1. Background
Pseudomonas aeruginosa is an important pathogen that is frequently involved in infections of weakened immune system (1). P.aeruginosa is the most common pathogen isolated from hospitalized patients and is a frequent cause of nosocomial infections such as pneumonia, urinary tract infections (UTIs), and bacteremia. Attempts of treatment of P. aeruginosa from patients through intense antimicrobial therapy may lead to significant selection of resistance strains in care units of the hospitals (2).
B-lactamases are enzymes produced by bacteria that inactivate β-lactam antibiotics. Extended-spectrum β lactamases (ESBLs) have the ability to hydrolyze and cause resistance to various types of the modern β-lactam antibiotics. Many P.aeruginosa strains can produce different classes of ESBLs that enable organisms to resist against many cephalosporins, such as cefotaxime, ceftriaxone, and ceftazidime (3). ESBLs were discovered in Europe in the early 1980s (4). Now, four classes are recognized, classes A, C, D, and class B or metallo-β-lactamases that need zinc for their action (3). These enzymes are encoded by chromosomal or plasmid-born genes, that are inactivated by the β-lactamases inhibitor such as clavulanate (5-7).
ESBL enzymes traditionally have been derived from TEM and SHV parent enzymes. The OXA-type ESBLs have been found mainly in P. aeruginosa isolates in Turkey and France (8). However, recently several classes such as A, B, and D ESBLs have been reported in P. aeruginosa. These beta-lactamases differ from the TEM and SHV enzymes. While most ESBLs have been found in Escherichia coli, Klebsiella pneumoniae, and other Enterobacteriaceae, the OXA-type ESBLs have been found mainly in P. aeruginosa isolates (9).
2. Objectives
This study was done to detect the frequency of OXA10 β-lactamase gene in MDR P. aeruginosa by PCR assay.
3. Materials and Methods
3.1. Bacterial Sources
Over 100 clinical samples including urine, blood, wound, sputum, and respiratory secretions were collected from hospitals in Isfahan within five months period (February to June 2012). Identification of the isolates was done according to standard microbiology tests including colonial morphology, oxidase positivity, the presence of characteristic pigments, and growth at 42°C (9, 10).
3.2. Antibiotic Susceptibility Pattern
Antibiotic discs included ciprofloxacin (from fluoroquinolones), Ceftazidime, Ceftriaxone, (from cephalosporins), Gentamicin, Tobramycin, Amikacin (from aminoglycosides), Imipenem, Cefepime (from carbapenems), Oxacillin (from penicillins), Piperacillin, and Cefotaxime (Himedia, India). Antimicrobial susceptibility tests were performed by the disc diffusion method according to the National Committee for Clinical Laboratory Standards (NCCLS) guidelines. P. aeruginosa PTCC 1310 was used as quality control strain in susceptibility determination. Multidrug-resistant (MDR) isolates were defined as those showed resistant to three or more classes of antipseudomonal agents (carbapenems, fluoroquinolones, penicillins, cephalosporins, and aminoglycosides) (8-10).
3.3. DNA Extraction
One ml of P. aeruginosa fresh cultures in Brain-Heart Infusion Broth (BHI) medium (Scharlau, Spain) were transferred into 1.5 mL sterile microfuge tubes and centrifuged at 13,000g for 10 minutes. The pellets were dissolved in 500 μl of lysis buffer (NaCl 1 M, Tris-HCl 100 mM, EDTA 0.5 M), SDS (10%) and 3μl of proteinase–K (20 mg/mL), and incubated at 55ºC for 2 hours. After the lysis, 500 μl of phenol/chloroform/isoamylalchol (25:24:1 Volume/Volume) were added to the above solutions, mixed by vortex, and then centrifuged at 13,000 g for 10 minutes. The supernatants were transferred to other sterile microfuge tubes. 1 ml of 95% cold ethanol was added and then they were left to stand for 1hour in refrigerator (4◦C). DNA was then precipitated in each tube by centrifugation at 12,000 g for 10 minutes. The precipitated DNA was dissolved in 50 μl of 10 mM Tris EDTA buffer (TE) as described by Sambrook et al., and used for further investigation (11).
3.4. PCR Reaction
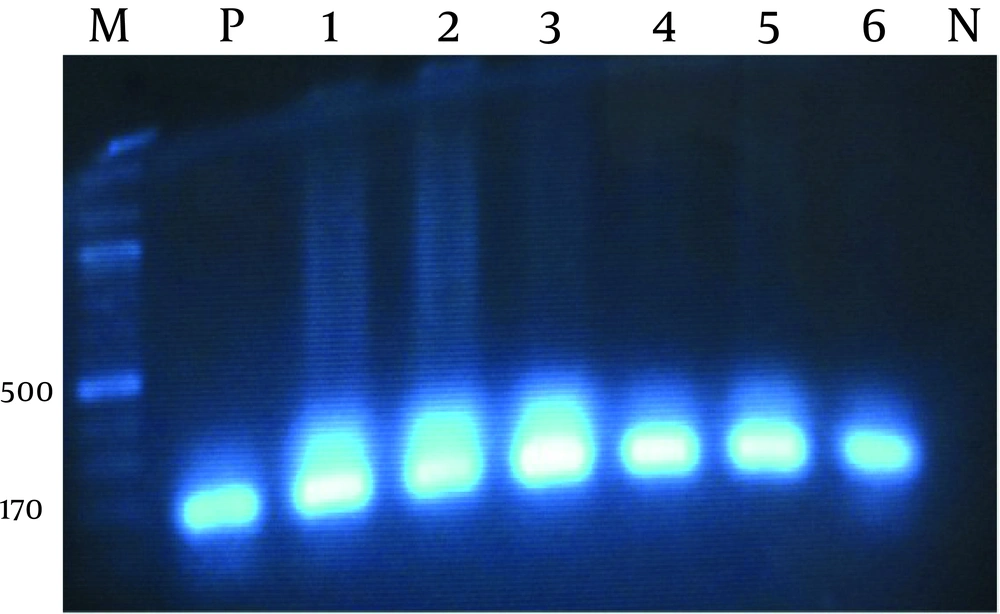
PCR was used to detect OXA10 gene in the multidrug resistant bacterial strains using the following primers designed (in this study): PaOxa10 F (5ATTATCGGCCTAGAAACTGG-3 And PaOxa10 R (5-CTTACTTCGCCAACTTCTCTG-3). PCR was carried out with 2µL of the template DNA, 0.4 pM of each primer, PCR buffer 1x , 200 µM dNTP , 1/5 mM MgCl 2 and 1 U of Taq DNA polymerase (Sinaclon, Iran) in a total volume of 50 µl. Amplification was carried out in a thermocycler (Eppendorf Mastercycler®, Massachusetts, USA). Agarose gel electrophoresis (1%) of PCR products was carried out using 1 mM Tris-Borate-EDTA (TBE) buffer at 95V for 1 hour, and then the DNA bands were stained with ethidum bromide (Sinaclon Iran). 100 bp DNA ladder was used to confirm the size of the specific bla gene.
Simultaneously, a positive control was used for blaOXA10 gene. The reaction conditions were as following: pre-denaturation at 94°C for 4 minutes, followed by 35 amplification cycles of 94°C for 1 minute, 55°C for 1 minute and 72°C for 1.5 minutes, with a final extension step of 72°C for 5 minutes. Sequencing of the PCR products: DNA sequencing for strains was performed for identification of detected bla gene using primers as described, and subjected to direct sequencing of both strands performed by the Macrogen company (Seoul, Korea), ( 12 ). The nucleotide sequences were analyzed with CROMAS and MEGA-4 softwares (Figure 1).
Statistical Analysis SPSS version 11.5 was used to analyze the data, and a Fisher exact test was used for the categorical data. P value of ≤ 0.05 was considered significant.
4. Results
Total of 100 clinical isolates of P. aeruginosa were collected from different samples (February to June 2012), where 65% of the isolates were from urine, 3% were from ear mucus, 20 % were from wounds, and 12% were from other samples. Using disk diffusion method, resistance rates are shown in Table 1. Our results reported the highest resistance rate to amikacin, oxacillin, cefotaxime, with 65%, 100 %, and 62%, respectively; and lowest resistance rate to piperacillin, imipenem, cefepime with 48%, 55%, and 55%, respectively. In present study resistance to gentamicin 59%, tobramycin 61%, ceftazidime 57%, ceftriaxone 60%, and cefotaxime 62%, was observed. The 52 isolates were sensitive to piperacillin.
aAbbreviation: CIP, Ciprofloxacin; CTX, Cefotaxime; CAZ: Ceftazidime, TOB, Tobramycin; AN, Amikacin; GM, Gentamicin; IPM, Imipenem; FEP, Cefepime; OX, Oxacillin; PIP, Piperacillin; CRO, Ceftriaxone.
Also PCR was performed for all the resistant strains, where the frequency of bla OXA10 gene was 64% among 63 strain with multidrug resistance (Figure 2), which have been a significant increase compared to previous studies. There was 13 (36%) isolates that were resistant to three antibiotics (cefotaxime, ceftriaxone, ceftazidime), but did not carry the blaOxa10 gene. All of isolates were resistant to Oxacillin, while 40 (64%) were carriers of this gene. It could be said that in the present study, most of the urine samples with multiple resistance were producers of bla OXA10 B-lactamase.
5. Discussion
The prevalence of multidrug-resistant P. aeruginosa producing ESBLs strains is increasing Worldwide (13). Plasmid or chromosomal mediated antibiotic resistance is common in P. aeruginosa isolated from patients in different hospitals in Iran (14). In our study, amikacin resistance value was higher than the values reported in the study conducted by Amutha et al.(15). In the study of Prashant et al. (16), the highest resistance rate was to ceftazidime (53%) and the lowest resistance rate was to imipenem (12%). Thus, our results are different from the results in India. Strateva et al. (17), reported resistance rate to ticarcillin 53% and clavlanic acid 22%. Lee et al (18), studied 252 isolates where 53 (21.0%) isolates carried OXA-type enzymes. Among those, blaOXA-10 was most prevalent, followed by OXA-4, OXA-2, OXA-30, and OXA-17. Six isolates (2.4%) carried two different b-lactamases and one carried three enzymes. (18).
Bert et al. reported that four isolates produced ESBLs according to the double disc synergy test. PCR detecting sequences for enzymes of the OXA-10 group was positive in 68 (26.3%) isolates; where 31 isolates carried blaOXA-10, 1 isolate carried blaOXA-14, and 36 isolates carried a new variant intermediate between blaOXA-13 and blaOXA-19. The blaOXA-2 gene was identified in 13 (5%) isolates (17). Lee et al. (18), found that in 64 (25.4%) isolates, the frequency of structural genes including OXA-10 was 13.1%, OXA-4 was 4.3%, OXA-30 was 2.0%, OXA-2 was 2.3%, and OXA-17 was 0.4%; where their distribution varied between provinces. Suk et al. (19), reported that 2.9% of isolates were multidrug-resistant (MDR), where PCR amplification with specifically designed primers and direct sequencing of the PCR products showed that the blaOXA-10, blaVIM-2, blaOXA-2, blaOXA-17, blaPER-1, blaSHV-12, and blaIMP-1 genes were carried by 34.3%, 26.9%, 3.0%, 3.0%, 1.5%, 1.5%, and 1.5% of 67 MDR P. aeruginosa isolates, respectively (20). The most prevalent beta-lactamase genes were blaVIM-2 and blaOXA-10 in the study of Suk et al. (19). Prevalence of blaOXA10 in the study of Shahcheraghi in Tehran was 92% in burnt patients (21).
Finally, the prevalence of MDR P. aeruginosa encoding blaOxa10 in Iran (64%) was five-fold higher compared with from Korea (13%), and also higher than from France (26%) and India. The prevalence of this gene in Tehran (92%) is higher than in our study, where this is probably due to the high prevalence of these resistant genes in burnt patients. According to results of present study, it can be concluded that bla OXA-10 gene has a high frequency in the P. aeruginosa strains isolated from hospitalized patientsin Iran compared to another countries such as France in 2002, India in 2011, Korea in 2005, and China in 2006.