1. Background
Urogenital infections are caused by Chlamydia trachomatis worldwide (1). This bacterium is the most prevalent cause of bacterial sexually transmitted diseases (STD). The prevalence of these infections can be different depending on the country and population type. The prevalence of C. trachomatis infection among sexually active women in developing countries is higher than developed countries (2, 3). Annually, about one million C. trachomatis infections are reported among sexually active young women in the united states, according to the Center for Disease Control and Prevention (CDC) (4). Most of the infected individuals are asymptomatic; so, the majority of these infections are not detected and treated, and this can lead to pelvic inflammatory diseases, with bacteria reaching the upper genital tract of the infected women (5).
The etiological relation of pelvic inflammatory diseases (PIDs) with microorganisms is not completely determined; but, the presence of a variety of microorganisms including C. trachomatis, Mycoplasma genitalium, Neisseria gonorrhea and some other microorganisms have been shown in lower and upper genital tracts of women with PID (6-11). Nevertheless, C. trachomatis is more suspected than other sexually transmitted diseases to causes symptomatic PID (9, 12, 13). PID can induce disorders such as infertility and ectopic pregnancy. These disorders may be the results of destruction of cilia layer of the fallopian and closure of fallopian tube (14). Therefore, detection and control of C. trachomatis infection prevalence is necessary to prevent its related sequels.
The duration of tubal inflammation and damages resulted from infection are two important factors affecting the efficiency of Chlamydia control programs. Therefore, it is important to detect and treat the infection before its development to short-term sequels such as pelvic inflammatory diseases, which in turn can develop into long-term sequels such as tubal factor infertility and ectopic pregnancy (15). In Iran, there are low epidemiological data regarding the prevalence of C. trachomatis infection and its consequences and it is clear that having more epidemiological knowledge about genital C. trachomatis infection prevalence could be very effective in choosing efficient strategies for screening and treatment of such infections.
2. Objectives
The purpose of this research was to determine the prevalence of C. trachomatis in healthy women and women with infertility disorder to evaluate the association between C. trachomatis infections and infertility in Iran.
3. Materials and Methods
3.1. Population
This research was a case-control study with 350 female participants. The case group consisted of 150 infertile women, 20 to 40 years, with no recognized physiological deficiency for infertility (unknown cause). The control group consisted of 200 impregnated women, 20 to 40 years, with one or more successful childbirths and without any history of infertility (Table 1).
| Age Groups | Case Group (n = 150) | Control Group (n = 200) |
|---|---|---|
| 20 | 31 | 31 |
| 20 - 24 | 23 | 55 |
| 25 - 29 | 80 | 93 |
| 30 - 34 | 10 | 12 |
| 35 - 39 | 4 | 5 |
| > 40 | 2 | 4 |
| Age average | 24.3 | 25.2 |
3.2. Specimen Collection
Two types of specimens from the both case and control groups were used in this study including endocervical mucosa and blood serums. Sampling from endocervical mucosa was performed using the Pap smear method.
3.3. Laboratory Methods
Direct and indirect immunofluorescence methods were used for determining the presence or absence of C. trachomatis infection in both case and control groups. For direct testing, endocervical swabs were transferred on clean slides and the slides were incubated at room temperature for drying and fixation. One drop of antichlamydial monoclonal antibody (antibody-online, USA) was added to each slide and they were incubated at 37°C for 10 minutes. Then, the slides were washed with phosphate buffered saline (PBS) and distilled water for 10 and 5 minutes, respectively. Finally, the slides were examined for C. trachomatis by fluorescence microscopy (Micros, Austria).
Using this technique, the elementary bodies are observed as fluorescence green spots. For indirect testing, C. trachomatis serotypes D-K, L1-L3 and IOL-207 (TWAR) were used as standard antigens. The serotypes were individually grown in eggs and then placed on specific areas of the slides. A spot on the slide was also specified for the mixture of egg yolk in PBS (PAA, Holland) as a negative control. Serums in dilutions ranging from 1:16 to 1:256 were prepared and individually added to the slides. After 30 minutes of incubation at 37°C, the slides were washed and stained with fluorescence antihuman globulin. Finally, the slides were examined by fluorescence microscopy and the results were recorded based on their color and fluorescence intensity (Table 2).
| Fluorescence Intensity and Color | Positivity or Negativity |
|---|---|
| Very shiny green | +++ |
| Shiny green | ++ |
| Green | + |
| Yellowish green | ± |
| Orange | - |
3.4. PCR Mixture and DNA Amplification
Endocervical swab samples were obtained from the patients. Each swab was placed in a sample collection vial containing the buffer (PBS). Several methods are available for DNA extraction. In the present study, we used the boiling method. We selected one pair of oligonucleotide primers (Cinnagen, Iran) specific for a region of the C. trachomatis gene (accession No. AB695165.1), coding for the major outer membrane protein (MOMP). The sequences from 5' to 3' of these oligonucleotide primers are as follows:
Forward: 5'-CCTGTGGGGAATCCTGCTGAA-3'
Revers: 5'-GTCGAAAACAAAGTCACCATAGTA-3'
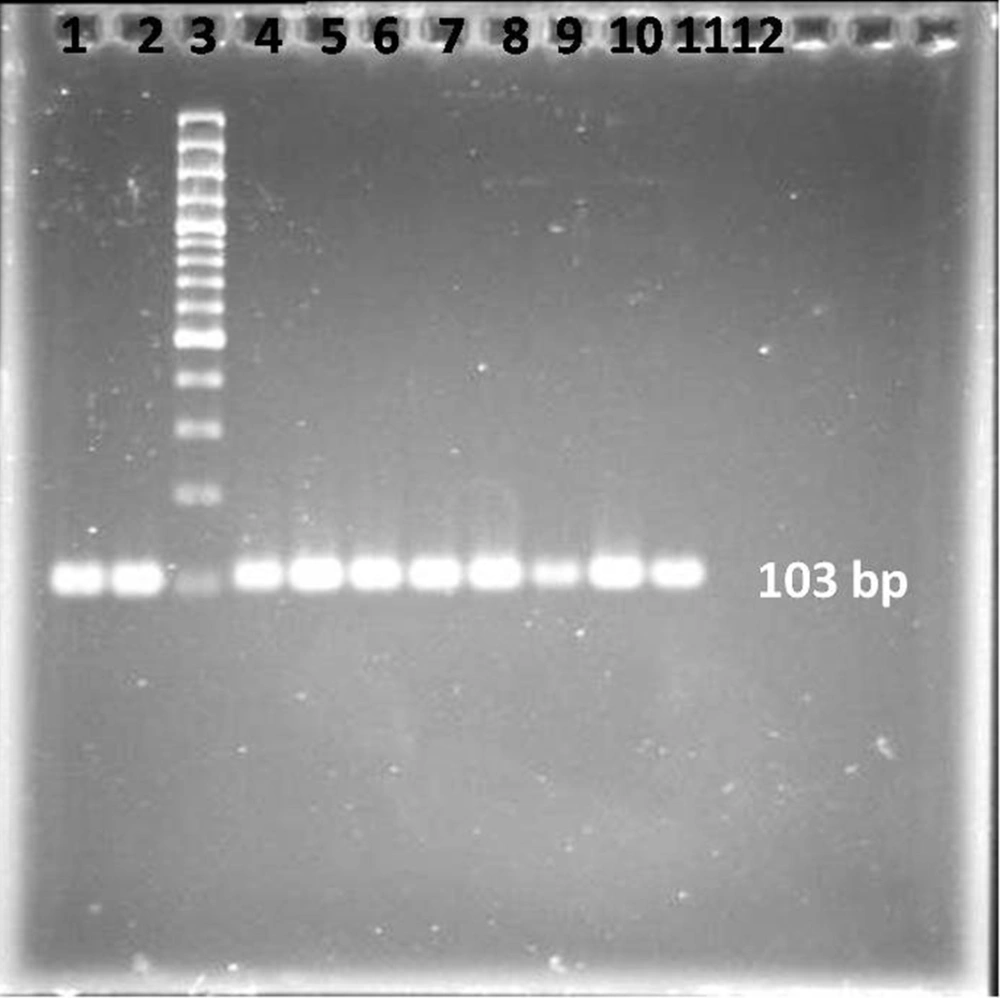
A master mixture of these reagents was made for the samples along with the positive and negative controls. The final reaction mixture of 50 µL for each sample contained 0.5 µM of each primer; 100 µM of each of dATP, dCTP, dGTP and dTTP; 50 mM KCl; 10 mM Tris-HCl, pH 8.3; 1.5 mM MgCl2, 1.25 units of Taq DNA polymerase enzyme, and 9 µL of the sample DNA. All the PCR reagents were purchased from the SinaClon company, Iran. Each microfuge tube containing the PCR mixture of 50 µL was mixed and subjected to 40 cycles of amplification. Each cycle composed of sequential incubations at 94°C for 1 minute for DNA denaturation, 52°C for 1 minute for annealing the primer to the templates, and 72°C for 30 seconds for DNA chain extension. At the end of the 40 cycles, the samples were kept for another 7 minutes at 72°C for completion of the extension of a DNA chain. The PCR product samples were immediately frozen for further analysis. The PCR products were visualized by agarose gel electrophoresis. A 10-µL post-PCR mixture was subjected to electrophoresis on 2% agarose gel (SinaClone, Iran) in the presence of ethidium bromide (Sigma, Germany). A DNA ladder (SinaClone, Iran) was also run simultaneously to confirm the size of the amplified product (103 bp) (Figure 1). The DNAs were extracted from the bands on the gel using a gel extraction kit (Qiagen, GmbH, Germany) and then sequenced by Macrogen Inc, Seoul, Korea.
4. Results
The results of direct and indirect immunofluorescence tests are shown in Table 3. As they show, the prevalence of C. trachomatis in the case and control groups reached to 15.3% and 3.5%, respectively, using the direct immunofluorescence method. For the indirect immunofluorescence method, the prevalence of C. trachomatis reached to 22.6% and 4.5% in the case and control groups, respectively. In addition, the prevalence of C. trachomatis in the case and control groups reached to 32% and 8.7%, respectively, using PCR method. The P values for direct and indirect immunofluorescence and PCR results were calculated by the Fisher test where a P value < 0.001 for direct test results and P < 0.004 for indirect and PCR test results were obtained (Table 3).
| Anti-Chlamydial Antibody Titer | Case Group Positives (n = 150) | Control Group Positives (n = 200) | Fisher Test Results |
|---|---|---|---|
| Direct immunofluorescence | 23 (15.3) | 7 (3.5) | P < 0.001 |
| Indirect immunofluorescence | 10 (6.6) | 5 (2.5) | |
| 1:16 | |||
| 1:32 | 18 (12) | 2 (1) | |
| 1:64 | 6 (4) | 2 (1) | |
| 1:128 | 0 | 0 | |
| PCR | 48(32) | 13 (8.7) | P < 0.004 |
5. Discussion
C. trachomatis is one of the most prevalent causes of sexually transmitted diseases. It is the major cause of urethritis and cervicitis, as well as their sequels such as PID and tubal factor infertility (16). Generally, Chlamydial infections are more destructive for reproductive health of women than men (5).
Svenstrup et al. reported that 23% of women suffering from tubal factor infertility (TFI) had antibodies against C. trachomatis, compared with 15% of women in the control group with normal tubes (17). In another research, Siemer et al. showed considerably higher prevalence of IgG and IgA antibodies (39% and 14%, respectively) among women with infertility compared with members of the control group (19% and 3%, respectively) (18). In a study carried out by Malik et al. the presence of C. trachomatis in infertile women was 28.1%, which was significantly higher than that of healthy women (3.3%) (P < 0.01) (19). Gaudoin et al. reported that 91.2% of woman with tubal occlusion had IgG antichlamydial antibodies in their sera (20).
Our results were compatible with the above studies.
In our study, the presence of C. trachomatis infection in the 150 infertile women with an average age of 24.3 years as the case group as well as 200 healthy women with an average age of 25.2 years as the control group was examined using direct and indirect immunofluorescence and PCR methods. In the direct immunofluorescence method, 15.3% of the case and 3.5% of the control group members showed positive results. The indirect immunofluorescence test also determined that 22.6% of the case and 4.5% of the control group members had a titer ≥ 1:16. The obtained P values from the results of direct and indirect immunofluorescence experiments by Fisher test were P < 0.001 and P < 0.004, respectively, confirming the significant association between urogenital chlamydial infections and infertility. The difference between the results of direct and indirect immunofluorescence tests could be due to old chlamydial infections or infections in other areas of the body.
Our results suggested that there was a significant association between C. trachomatis infection and female infertility. Therefore, C. trachomatis can be one of the main ethological factors for female infertility. Consequently, it is recommended that infertile women without any physiological deficiency should be examined for contamination with C. trachomatis. Finally, for detection of genital C. trachomatis, PCR results are more reliable than immunofluorescence tests.