1. Background
Unlike other prokaryotes, the class mollicutes lack the cell wall and with unusually different genome are relatively small in size. The largest group is formed by the genus mycoplasma of which more than 90 species have been described (1). Mycoplasmatales are associated with infection of the genitourinary tract, reproductive failure, and neonatal morbidity and mortality. Infection with genital mycoplasmas has been linked with infertility (2-4). Ureaplasma spp. are the main cause of non-chlamydial, non-gonococcal urethritis and acute prostatitis (2). In pregnancy, Ureaplasma can cause chorioamnionitis and preterm delivery (5).
Mycoplasma homonis has been associated with pyelonephritis, pelvic inflammatory disease and postpartum septicemia (2). Diagnosis of mycoplasmal infections is usually made by serological determination or by culture. However, serological procedures are often hampered by interspecies cross reactions, and cultivation is costly since it requires special media and expertise. It can take 2-5 days to culture Ureaplasma spp. and M. hominis (2, 6). The need for an improved detection method for M. hominis and Ureaplasmaurealyticum is evident. Polymerase chain reaction (PCR) to amplify specific short segments of nucleic acid sequences is a promising rapid diagnostic test (7, 8).
2. Objectives
The current study aimed to develop the multiplex PCR assay to detect two genital mollicutes from a single amplification reaction and the study of their relation with habitual abortion and urogenital infection in infected females.
3. Materials and Methods
3.1. Bacterial Strains
The following microorganisms were purchased from the American Type Culture Collection (ATCC) and National collection of type cultures (NCTC): U. urealyticum (NCTC 10177T) and M. hominis (ATCC 23114). These microorganisms were used as positive controls.
3.2. Clinical Specimens
All specimens were received in the clinic of Imam Khomeini hospital. Specimens included 155 cervicovaginal swabs from females with vaginal discharges or genital infections and 110 urine samples from females with urine infections. Also control urine and swab samples (100 of each) were obtained from asymptomatic females. The symptomatic subjects completed standardized questionnaires about their age, educational status, any symptoms of urogenital infection and history of abortion. The swabs were transported to the laboratory in mycoplasma transport medium (PPLO broth, Difco, USA). Approximately 20 ml of clean-catch urine was then collected in a sterile plastic container. Urine samples concentration increased 10 fold by centrifugation for 30 min at 1600 ×g prior to testing. All of the specimens were stored at-70°C until DNA extraction (2).
3.3. DNA Extraction
DNA was extracted from standard strains and clinical samples as described previously by Cadieux et al. 1999 (9). Briefly, 1 ml of the sample was centrifuged at 12000 ×g for 10 min. The pellet was washed in PBS buffer and resuspended in 30 µl of dH2O. After boiling for 10 min, an aliquot of 7 µl was used directly in PCR experiments as DNA template (9).
3.4. Multiplex PCR Assay
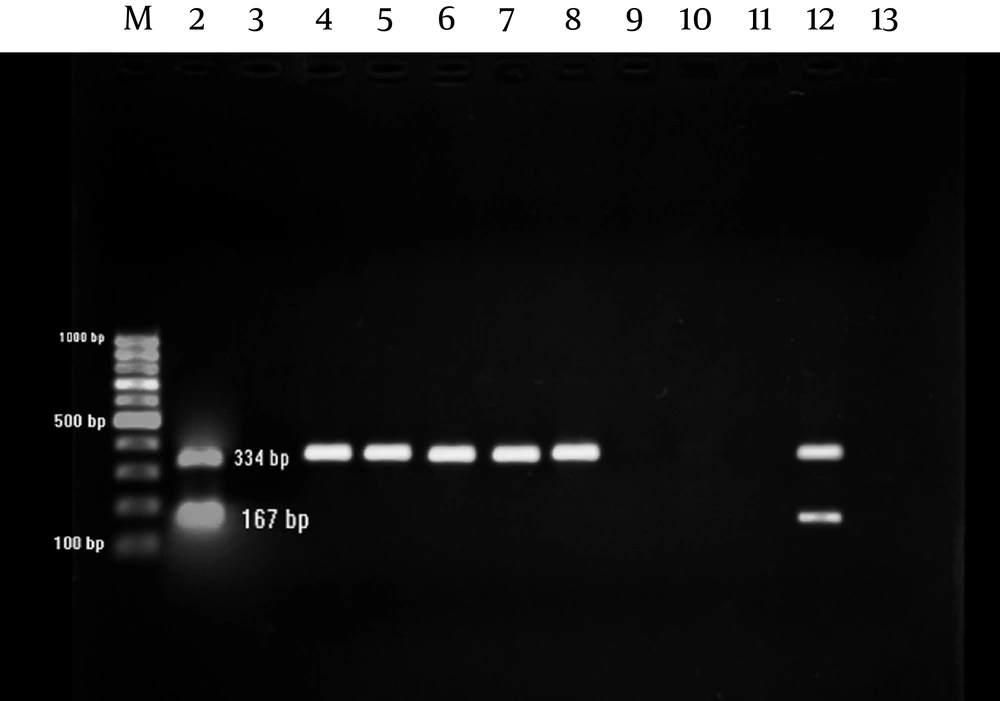
The sequences of primers for each bacterium are listed in Table 1. Multiplex amplification reaction was prepared in 25 µl final volume containing: 10 ρM of each RNH primer and 5 ρM of each U primer (Tuba Negin, Tehran, Iran), 1.5 U Taq DNA polymerase, 1× PCR buffer, 0.2 mM dNTPs, 3 mM MgCl 2 (Cinagene, Iran), 0.1-0.5 µg DNA template and water up to 25µl. The PCR amplification was carried out using a thermal cycler (Biorad, USA) with thermal profile as follow: initial denaturation step at 95ºC for 4 min, 35 cycles at 95ºC for 50 sec, annealing temperature at 53.7ºC for 50 sec, extension step at 72ºC for 60 sec, followed by final extension at 72ºC for 7 min. The PCR products were visualized and photographed under UV light after electrophoresis for 45 min at 100 V through 1% agarose gel containing ethidium bromide (1 µg/mL).
| Analysis, organism, and primer | Target or DNA sequence (5'-3') | Length, bp | Reference |
|---|---|---|---|
| Multiplex PCR | |||
| U. urealyticum | Urease gene | 167 | (10) |
| U9 primer | GAG ATA ATG ATT ATA TGT CAG GAT CA | ||
| U8 primer | GAT CCA ACT TGG ATA GGA CGG | ||
| M. hominis | 16S rDNA | 334 | (11) |
| RNH1 primer | CAA TGG CTA ATG CCG GAT ACG C | ||
| RNH2 primer | GGT ACC GTC AGT CTG CAA T | ||
3.5. Statistical Analysis
Statistical analysis was performed by SPSS statistical software package V.11.5 and Chi-Square test. Statistical significance was assumed at the P<0.05 level.
4. Results
M. hominis and U. urealyticum were detected by amplifying 334 and 167 bp amplicons, respectively; using multiplex PCR (Figure 1).
M. hominis was detected in 6.4% of urine samples collected from symptomatic but no asymptomatic women (P=0.01) and in 7.1% of genital samples from symptomatic but not from asymptomatic women (P=0.006) (Table 2).
| Bacteria | Genital samples | Urine samples | ||||
|---|---|---|---|---|---|---|
| Case group, n = 110, No. (%) | Control group, n = 100, No. (%) | P Value | Case group, n = 155, No. (%) | Control group, n = 100, No. (%) | P Value | |
| M. hominis | 7 (6.4) | 0 (0) | 0.01 | 11 (7.1) | 0 (0) | 0.006 |
| U. urealyticum | 15 (13.6) | 8 (7.2) | 0.191 | 13 (8.4) | 5 (3.2) | 0.303 |
| Total | 22 (20) | 8 (7.2) | - | 24 (15.5) | 5 (3.2) | - |
In urine samples, the highest frequencies of M. hominis and U. urealyticum were observed in females within 30-34 years old (71.4%) and 35-39 years old (60%), respectively (Table 3). On the other hand, in genital samples, the highest frequencies of M. hominis and U. urealyticum was observed in females within 28-33 years old (54.5%) and 34-39 (53.8%), respectively (Table 3).
In addition, the relation of habitual abortion with the presence of the studied mollicutes in either urine or genital samples was also analyzed. Surprisingly, the incidences of both mollicutes were considerably higher in urogenital samples among symptomatic females with habitual abortion history in comparison with those with no habitual abortion history (Table 4). M. hominis and U. urealyticum were recovered in 33.3% and 66.7% of genital samples, respectively; from symptomatic females with habitual abortion history (P < 0.001). Similar results were also obtained in the case of urine samples (Table 4).
| Urine samples | Genital samples | ||||
|---|---|---|---|---|---|
| Age group, y | M. hominis, No. (%) | U. urealyticum, No. (%) | Age group, y | M. hominis, No. (%) | U. urealyticum, No. (%) |
| 20-24 | 0 (0) | 1 (6.7) | 16-21 | 1 (9.1) | 1 (7.7) |
| 25-29 | 1(14.3) | 2 (13.3) | 22-27 | 2 (18.2) | 3 (23.1) |
| 30-34 | 5 (71.4) | 2 (13.3) | 28-33 | 6 (54.5) | 1 (7.7) |
| 35-39 | 1 (14.3) | 9 (60) | 34-39 | 1 (9.1) | 7 (53.8) |
| 40-44 | 0 (0) | 1 (6.7) | 40-45 | 0 (0) | 1 (7.7) |
| 45-49 | 0 (0) | 0 (0) | 46-51 | 0 (0) | 0 (0) |
| 50-54 | 0 (0) | 0 (0) | 52-57 | 0 (0) | 0 (0) |
| - | - | - | 58-63 | 1 (9.1) | 0 (0) |
| Total | 7 (100) | 15 (100) | Total | 11 (100) | 13 (100) |
| Samples | Habitual abortion, No. (%) | M. hominis | P Value | U. urealyticum | P Value |
|---|---|---|---|---|---|
| Genital | 15 (9.7) | 5 (33.3) | P< 0.001 | 10 (66.7) | P< 0.001 |
| Urine | 10 (9.1) | 2 (20) | P = 0.014 | 7 (70) | P < 0.001 |
5. Discussion
The role of mycoplasmas in the genital and extragenital systems is speculative and depends on epidemiologic data ( 6 ). Clinical studies showed that mycoplasma incidence is raised in the presence of an anaerobic primer pathogen such as Trichomonas vaginalis, Chlamydia trachomatis or Neisseria gonorrhea ( 6 ). In current study, statistical analyses revealed the correlation between the incidence of M. hominis in symptomatic as well as asymptomatic females with urine (P = 0.01) and genital (P = 0.006) infections (Table 2), while no significant relation was observed between the incidence of U. urealyticum and urine (P = 0.191) and genital (P = 0.303) infections.
Mycoplasmas can grow in the stress environment created by primary pathogen. Notably, these microorganisms colonize numerously in sexually active women, but they cannot be detected due to less sensitivity of microbiological cultivation methods unless an infection occurs (11). Based on the literature, diseases such as pelvic inflammatory disease, infertility (12), habitual abortion (13), bacterial vaginosis (14), cervicitis (15), non-gonococcal urethritis (7) and chorioamnionitis (5) have been reported to be associated with M. hominis and U. urealyticum infection. Therefore, if these microorganisms are really pathogens, their early detection would be of high value. Hence, a sensitive, specific, fast, cheap and easy applicable diagnostic method is necessary.
The current study found, M. hominis and U. urealyticum in urogenital infected samples with the incidence of 6.4 to 13.6%. However, studies in other countries showed that the incidence of M. hominis and U. urealyticum was relatively higher (6, 16). Since M. hominis and U. urealyticum have been found significantly associated with low socioeconomic background, the lower incidence in the current study is not surprising, considering the low number of sexual partners among Iranians, limitations in sexual relationships for non-married people and public awareness on using contraceptive drugs (17, 18). The highest incidence of M. hominis and U. urealyticum was observed in females with urogenital infections between 30-39 years old all of whom were sexually active.
These results support previous reports on the presence of M. hominis and U. urealyticum in sexually active adults (10, 11). Apparently, the level of colonization of genital mycoplasmas is highly affected by fluctuation of estrogen and progesterone hormones (19). In addition, within this range; more sexual activity, desired condition of urogenital tract mucosa and utilization of contraceptive pills cause higher level of colonization of mycoplasmas in comparison with those of non-sexually active adults (11, 20). In the current study, 25 out of 265 females with urogenital infections had history of habitual abortion. Statistical analyses showed the direct relation between the presence of M. hominis,U. urealyticum, and habitual abortion (P < 0.001). This result was in agreement with previous report on habitual abortion in the presence of these mollicutes (13).
The current study found strong relation between the presence of the studied M. hominis and U. urealyticum with urogenital infection in females in comparison with those of the control groups. In addition, it was shown that the studied mollicutes were highly associated with habitual abortion in symptomatic females. Eventually, the multiplex PCR in the current study was developed for simultaneous, early and easy detection of these potential pathogens.