1. Background
Physical, chemical and biological properties of indoor air can affect health and welfare (1, 2). Indoor air quality is not easy to determine and control and may result in poor air quality for health workers and people involved in these environments (3). Indoor air of hospitals has a wide range of infectious microorganisms (3, 4). The microorganisms load from one department to another in a particular hospital, as well as distinct hospitals in a city or region can vary (5). Estimating density and diversity of microorganisms in the air of a hospital can be an indicator of whether such environments are dirty or clean. In addition, it is considered as a source of hospital-associated infections (6).
Microorganisms are the primary source of air contamination in indoor environments (7). Indoor air has a greater potential to endanger patients health than outdoor air. Indoor aerosol types may have the ability to cause different levels of infection. Although many present biological substances in inhaled air are not considered as pollution but if their amount increases by several folds of their ambient amount, they can stimulate or poison people once inhaled (8). This pollution type includes materials such as air-borne particles, large molecules or volatile compounds that are both alive and released by living creatures. Some bioaerosols such as bacteria and viruses can multiply. Some others, such as pollen of plants and mite droppings may just be irritating (9, 10).
Motions of bioaerosols in the air depend on gravity, electromagnetism and the turbulence of air. Bioaerosol is a suspension of airborne particles that generally include live and dead bacteria or may be released from living organisms. Also bioaerosols can contain a variety of pathogenic and non-pathogenic viruses, fungi, high molecular weight allergens, endotoxins, fungal toxins, peptidoglycans, fungal spores and plant pollens (9, 10). Bioaerosols can be released through dust, dirt and water droplets in the environment (11). Contact with bioaerosols in different environments is associated with a risk of respiratory infectious diseases, acute toxic effects, allergies and cancer. Among indoor environments where bioaerosols are considered a problem, hospitals are of major concern as in these environments there are a wide range of people such as hospital and medical staff, service users, patients and visitors who can contact bioaerosols and inhale them (11, 12). Therefore, the presence of excess bioaerosol in hospitals air can be a serious health threat (13).
Perdelli et al. (14) studied fungi load in different wards of a hospital. They reported an average concentration of 19 ± 19 cfu/m3for fungi, where lowest concentration (14 ± 12 cfu/m3) was found in the operating room and highest concentration (45 ± 37 cfu/m3) in the kitchen. Fugal genera that had the greatest number were Cladosporium spp., Aspergillus spp., Penicillium spp. and Rizopous, respectively. In a study conducted by Jabbari et al. (15) highest concentration of bioaerosol was reported for the infectious diseases ward (300 cfu/m3) and the lowest mean concentration of bioaerosol was found at the Ear, Nose and Throat (ENT) and eye surgery wards (94 cfu/m3). The highest percentages of fungi found in the air of hospitals were Penicillium spp. (36.36%), Cladosporium spp. (24.74%), Aspergillusniger (17.97%), Rizopous (10.57%) and A. flavus (2.74%), respectively. As some previous studies have shown, exposure to a high density of bacterial bioaerosols can cause asthma and rhinitis (16, 17), hypersensitivity pneumonitis (17) and sick building syndrome (16). Among bacterial bioaerosols, those such as Bacillus sp., Streptomyces albus, Pantoeaagglomerans, Pseudomonas chlororaphis, Arthrobacterglobiformis, Thermoactinomycetes vulgaris, and Corynebacteriumsp. have potential allergenic or immunotoxic characteristics and are a probable cause of airborne infectious illnesses.
Most bioaerosols are non-pathogenic and only cause infection or illness in people with impaired or susceptible immune systems (15). Many researchers have confirmed that hospitals (all of wards) as a source for propagation and transmission of acquired infections and bioaerosols are at high risk. Therefore, knowledge about the prevalence of bacterial flora in hospitals and understanding types of infections and allergies caused by bioaerosols is of utmost importance. Microbial control in hospital environments and its air can also play a major role in the prevention of cross infection.
2. Objectives
This study obtains quantity of air pollution in view of bioaerosols, so it can show pollution levels and we can compare it with the guidelines, standards and similar studies that will help in understanding of contamination; as quantitative studies, due to say pollution levels and compare that with the guidelines, standards and similar studies will help in understanding of contamination; the aim of this study was to determine the density and diversity of bioaerosols in educational hospitals of Hamedan in 2012.
3. Materials and Methods
This cross-sectional study was performed for five educational hospitals under supervision of Hamedan University of Medical Sciences in Hamadan, during 2012. Due to budget constraints, only 6 wards from each hospital were studied to determine density and type of bioaerosol. Selecting hospital wards took place according to services provided by each hospital, importance of ward according to the type of hospitalized patients and proposal of infection control and environmental health offices located in each studied hospital. Studied hospitals and their geographical locations are demonstrated by Figure 1.
3.1. Bioaerosols Density Determination
The filtration method was used for bioaerosol sampling (17). This is one of the methods recommended by the Bioaerosols Committee of American Conference of Industrial Hygienists (ACGIH) and has been utilized by Ghorbani et al. (9) and Mohammadian et al. (11). Used instruments in this phase of the study were: a pump sampler, Teflon filter holder with a 47mm diameter, sterile mixed cellulose ester filter with support pad (MCES; 0,45 μm pore size, S-Pak sterile, Merck Millipore), disposable sterile petri plates (M/s GSV Enterprises), Sabouraud dextrose agar and blood agar culture medium (Merck, Germany). Before sampling, all required equipment disinfected by 70% alcohol at first then autoclaved at the standard temperature and pressure for 30 minutes. Next, all instruments were taken to hospitals in sterile packages.
In the hospital and in selected wards, samplers were prepared and samples were obtained. To increase accuracy, a number of environmental and laboratory samples were collected as control samples. Previously, using ACGIH guidelines and doing some pre tests, 10 L/min and 30 min were selected as the sampling strategy. Sampling was done at 1.2 to 1.8 meters above the ground (1.5 m for all samples) with more than a meter away from walls and obstacles. Finally, 36 samples were taken from each ward. 18 samples were taken to check for fungal bioaerosol contamination and 18 samples for bacterial bioaerosols. Two sampling systems with same conditions were used for bacterial and fungal sampling, simultaneously.
Along with sampling, some parameters such as air temperature, humidity and air velocity (every 10 minutes) were measured and recorded in a prepared checklist. Before and after each sampling event the flow rate of the sampler was checked and calibrated using a rotameter (Skc Inc. Houston, UA). If the difference between the initial and final flow rates was greater than 10%, the sample was rejected. At the end of the sampling, with standard conditions, filters were reversely placed on Sabouraud dextrose agar and Blood agar culture medium (for fungal and bacterial bioaerosol, respectively). They were transferred to the laboratory at standard conditions. Inoculated plates were incubated at 37°C for 48-72h, after which type and number of colonies per plate was determined. On each plate labels were attached (including sample code, hospital name, department name, sampling point). Volume of sampled air was corrected based on pressure and temperature of the sampling wards (1, 2, 9). The volume of sampled air was corrected using the following equation: P1 V1/T1 = P2 V2/T2
Where P is atmospheric pressure, V is volume of sampled air and T is sampled air temperature. Where, one and two indices represent standard conditions and sampling conditions, respectively. Temperature and humidity at the sampling wards were measured using a digital hygro-thermometer (Testoterm, Germany) and the air velocity was measured using an anemometer (Utron, AM-4201) (Fisons Scientific, Loughborough, UK). All measurements were made in triplicates and the averages were used. With volume of sampled air and the number of colonies cultured, bioaerosols densities were calculated in terms of number of colonies counted in a cubic meter of air (cfu/m3).
3.2. Bioaerosols Type Determination
To assess cognitive bacteria, a microbiological expert evaluated all incubated plates in term of colony growth, morphology, color and appearance. For staining and microscopic examination of each sample, one slide was prepared. Bacteria were studied using their shape (cocci and bacilli) and by the Gram stain method. Plates containing fungus were incubated at 25 to 27°C temperatures for 72 to 120 hours. After incubation, numbers of formed colonies on the plates were counted. The isolated fungi were primarily identified by their colony morphology and microscopic structures. To achieve exact fungus identification, previously reported identification methods such as tested mount and slide culture were used (7, 15, 18). The results of the experiments were analyzed using the SPSS (ANOVA and t-test statistical analyses) and Excel software.
4. Results
In the present study 6 wards from each of the five educational hospitals in Hamadan i.e., 180 samples evaluated for bioaerosol analysis and bioaerosol concentration were reported in term of cfu/m 3 . For each sample, bacterial and fungal bioaerosols were identified. Average bioaerosol concentration based on each hospital and ward is presented in Table 1. Physical characteristic of sampled air were 25.83 ± 2.67°C, 35.32 ± 6.66 % and 1.52 ± 0.27 fts-1 for air temperature, humidity and air velocity in the sampling position, respectively. According to Table 1, greatest bioaerosol concentration was detected for the emergency ward of Besat hospital (24.3 cfu/m 3 bacterial bioaerosol) and lowest for the women 3 and emergency wards of Fatemieh hospital (3.3 cfu/m 3 fungal bioaerosol).
| Hospital Name | Studied Wards for Each Hospital | ||||||
|---|---|---|---|---|---|---|---|
| Farshchian Hospital | ward | isolation | emergency | eye | infectious | operation room | ICU |
| Mean bacterial | 7.8 | 17.8 | 11.1 | 18.9 | 13.3 | 12.2 | |
| Mean fungal | 16.7 | 16.7 | 15.6 | 11.1 | 7.8 | 6.7 | |
| Ekbatan Hospital | ward | isolation | women | CCU | emergency | operation room | nurses' station |
| Mean bacterial | 10 | 20.2 | 10 | 13.4 | 12.2 | 23.4 | |
| Mean fungal | 13.4 | 5.6 | 11.1 | 5.6 | 20 | 8.9 | |
| Fatemieh Hospital | ward | isolation | women 1 | women 3 | emergency | operation room | neonatal 1 |
| Mean bacterial | 13.2 | 20 | 12.3 | 13.3 | 7.8 | 13.4 | |
| Mean fungal | 14.2 | 34.4 | 3.3 | 3.3 | 5.5 | 3.4 | |
| Shahid Beheshti Hospital | ward | isolation | emergency | nurses' station | ICU | operation room | nephrology |
| Mean bacterial | 22.3 | 16.5 | 22.3 | 16.6 | 22.5 | 22.5 | |
| Mean fungal | 26.8 | 18.7 | 16.8 | 11.1 | 5.6 | 11.2 | |
| Bessat Hospital | ward | isolation | emergency | nurses' station | pediatric | operation room | ICU |
| Mean bacterial | 19.9 | 24.3 | 14.6 | 20.1 | 11.1 | 18.9 | |
| Mean fungal | 15.5 | 7.7 | 12.4 | 14.5 | 21 | 21.3 | |
Total averages of bioaerosol concentration for all surveyed hospitals were 160.6 and 12.56 cfu/m 3 for bacterial and fungal bioaerosols, respectively. There was no significant difference between the mean bacterial bioaerosol concentrations and studied hospital wards (ANOVA, P = 0.25), but there was a significant difference between fungal bioaerosol concentration and studied hospital wards (P < 0.001). To determine whether the obtained results for bioaerosol concentration in hospital wards, is acceptable or not based on available guidelines, it is necessary to compare them with the guideline values. Currently there is no definitive standard or guideline accepted by all institutions or organizations. Given values by some organizations are considered as guidelines or proposed standards. For the present study, 30 cfu/m 3 was used as a guideline value for comparison. In Table 2, the results for the overall density of bioaerosols are presented. In this table, values more than the proposed guideline are denoted by an underline. According to Table 2, the highest and lowest bioaerosol concentrations were in women1 and operation room wards of Fatemieh hospital (54.4 cfu/m 3 VS 13.3 cfu/m 3 ).
| Hospital Names | Studied Wards For Each Hospital | Total Average | |||||
|---|---|---|---|---|---|---|---|
| Farshchian Hospital | isolation | emergency | operation room | infectious | eye | ICU | |
| 24.5 | 34.4 a | 21.1 | 30 | 26.7 | 18.9 | 25.93 | |
| Ekbatan Hospital | isolation | emergency | operation room | women | CCU | nurses' station | |
| 23.4 | 19 | 32.2 a | 25.8 | 21.1 | 32.3 a | 25.63 | |
| Fatemieh Hospital | isolation | emergency | operation room | women 1 | women 3 | neonatal 1 | |
| 27.4 | 16.7 | 13.3 | 54.4 a | 15.6 | 16.8 | 24.03 | |
| Shahid Beheshti Hospital | isolation | emergency | operation room | ICU | nurses' station | nephrology | |
| 49.1 a | 35.3 a | 28.2 | 27.7 | 39.1 a | 37.7 a | 36.18 a | |
| BessatHospital | isolation | emergency | operation room | ICU | nurses' station | pediatric | |
| 35.4 a | 32 a | 32.1 a | 31.2 a | 27 | 35.6 a | 32.22 a | |
aUnderline and bold denote cases more than 30 cfu/m3
Results of the statistical analysis which compared the overall bioaerosol density in studied hospitals with 30 cfu/m 3 , showed no significant differences (P = 0.3) (Table 3).
| Hospital Names | Studied Wards for Each Hospital | ||||||
|---|---|---|---|---|---|---|---|
| Farshchian Hospital | studied wards | isolation | emergency | operation room | infectious | eye | ICU |
| Ventilation system | HVACa | Natural | HVAC | Natural | Natural | HVAC | |
| Disinfectant | White King | White King | White King | White King | White King | Deconexb | |
| Number of Bed | 6 | 15 | - | 34 | 30 | 8 | |
| Ekbatan Hospital | studied wards | isolation | emergency | operation room | women | CCU | nurses' station |
| Ventilation system | HVAC & Natural | HVAC & Natural | HVAC | HVAC & Natural | HVAC & Natural | HVAC & Natural | |
| Disinfectant | Deconex+ White King | Deconex+ White King | Deconex+ White King | Deconex+ White King | Salon+deconex | Deconex+ White King | |
| Number of Bed | 2 | 9 | 11 | 11 | 33 | - | |
| Fatemieh Hospital | studied wards | isolation | emergency | operation room | women 1 | women 3 | neonatal 1 |
| Ventilation system | HVAC & Natural | HVAC & Natural | HVAC & Natural | HVAC & Natural | HVAC & Natural | HVAC & Natural | |
| Disinfectant | Deconex+ White King | Deconex+ White King | Deconex+ White King | Deconex+ White King | Deconex+ White King | Deconex+ White King | |
| Number of Bed | 1 | 9 | - | 18 | 15 | 11 | |
| ShahidBeheshti Hospital | studied wards | isolation | emergency | operation room | ICU | nurses' station | nephrology |
| Ventilation system | HVAC | HVAC & Natural | HVAC | HVAC & Natural | HVAC & Natural | HVAC & Natural | |
| Disinfectant | Deconex+ White King | Deconex+ White King | Deconex+ White King | Deconex+ White King | Deconex+ White King | Deconex+ White King | |
| Number of Bed | 2 | 9 | 1 | 5 | - | 14 | |
| Bessat Hospital | studied wards | isolation | emergency | operation room | ICU | nurses' station | pediatric |
| Ventilation system | HVAC | HVAC | HVAC | HVAC | HVAC | HVAC | |
| Disinfectant | Deconex+ White King | Deconex+ White King | Deconex+ White King | Deconex+ White King | Deconex+ White King | Deconex+ White King | |
| Number of Bed | 1 | 27 | 6 | 23 | - | 26 | |
a Air Conditioning system
bdeconex® 50 FF
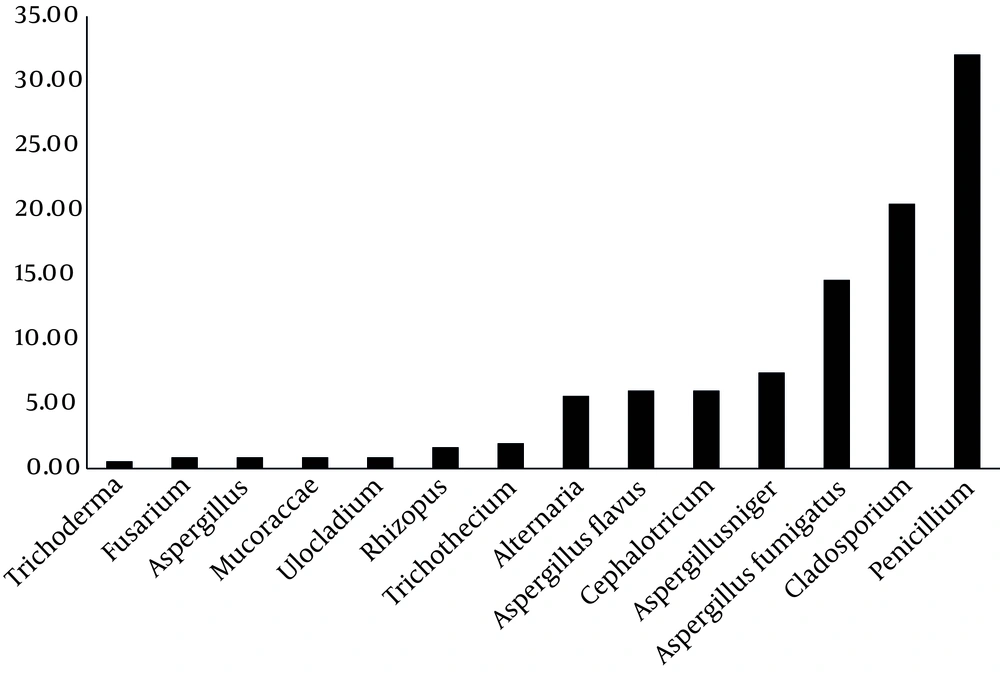
As Figure 2 shows, highest percentages of detected fungal genera are Penicillium spp. (32.06%), Cladosporium spp. (20.5%), A. fumigatus (14.61%), A. niger (7.43%), respectively.
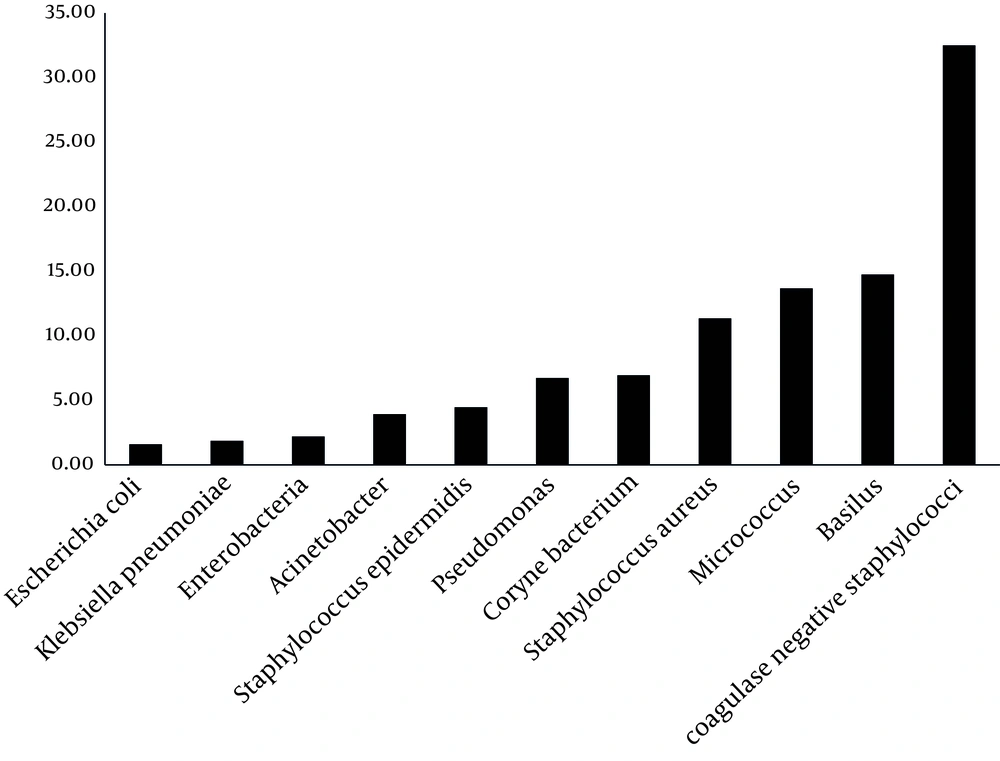
Based on the results presented by Figure 3, greatest percentage of isolated bacteria were coagulase-negative staphylococci (32.49%), Bacillus spp. (14.74%), Micrococcus spp.(13.68%) and Staphylococcus aureus (11.34%), respectively.
5. Discussion
In this study, 11 bacterial species and 14 fungal genera were identified from bioaerosol evaluation in surveyed hospitals. Comparison of diagnosed bioaerosol types by the present study indicates that the results are similar to previous studies. Jafal and colleagues (4) in their study identified S. aureus, Coccus, Micrococcus, Alpha-hemolytic Streptococcus, Diphtheroid bacilli, Gram negative bacilli and other bacillus genera, Streptomyces and a variety of bacteria and fungi from men, women, children, surgery, ICU and operation room wards, respectively. The results of the present study for identified bacterial species were similar to Jafal et al. bacteriological results. Their quantitative results of bacteriological studies revealed that children and women wards had the highest bioaerosol concentration, respectively. One of the probable reasons can be the high number of patients that refer to these wards (3).
In the present study, the highest bacterial bioaerosol concentration was found for the emergency ward of Bessat hospital (24.3 cfu/m3). The overall density of bioaerosol was the highest in women 1 ward in Fatemieh hospital (54.4 cfu/m3). In the emergency and women wards of hospitals there are some non-sterile devices such as personal belongings of patients and visitors, as well as overcrowding of patients and this may be the reason why the emergency ward may have a high diversity and density of bioaerosols (3). Similar studies have also noted these factors, e.g. Jabbari et al. (15) found women’s ward as the most contaminated. The researchers said that overcrowding of patient in this ward might be the main cause of these findings. In the present study, the most identified fungal genera was Penicillium spp. in the women’s ward, which is consistent with the Jabbari et al. study.
Abdollahi’s study (5) found that the quantity of fungal bioaerosol concentration in the ICU and coronary care unit, was higher than the other wards. They considered this as a risk factor for the sensitive patients admitted to these wards. In a study performed to assess fungal bioaerosol concentration of wards air carried out in Italy (14), Penicillium spp., Cladosporium spp. and Aspergillus were reported as the most common fungal bioaerosols, respectively. Penicillium and Aspergillus fungal bioaerosols were reported at 26 to 78 cfu/m3 for the studied wards. In general, we can say that the results of the present study are similar to other similar studies, qualitatively and quantitatively.
Bioaerosol density in the operating room has been minimal. This can be because of the high level of health standards, as well as disinfectant and air purification systems such as ultraviolet light application in this ward (3). The most frequent bacterial bioaerosol for all studied hospital wards was coagulase-negative Staphylococcus. Coagulase-negative staphylococci (CoNS) are part of the usual flora of human skin. These organisms have a relatively low virulence but are increasingly recognized as agents of clinically significant infection of the bloodstream and other sites especially for high-risk groups (19). Staphylococci are quite resistant to desiccation and high-osmotic conditions. These properties make their continued existence in the environment, growth in food, and communicability possible.
Botelho et al. (19) in their study isolated coagulase-negative staphylococci from hospital indoor air. They collected 108 coagulase-negative staphylococci (CoNS) from hospital indoor air. S. epidermidis (n = 27) and S. haemolyticus (n = 17) were the most frequent species identified. Thus they concluded that some airborne isolates display virulence profiles and levels of biofilm accumulation similar to those found in patient isolates. Hospital indoor air can be an important route for transmission of CoNS isolates. The most frequent species of fungal bioaerosols, identified by Perdelli et al. (14), from hospital indoor air were Cladosporium spp., Aspergillus spp., Penicillium spp. and Rhizopus, respectively. In addition, the most frequent species of fungal bioaerosols, identified by Panagopoulou et al. (20) among fungal genera, was Aspergillus spp.
Frequency and diversity of fungi in different published studies are not identical. Various factors such as the sampling season, impact of outdoor on hospital indoor air, type of admitted patient, type of ventilation system and its effectiveness, and efficiency of disinfection can affect frequency and diversity of isolated fungi from indoor hospital air. Kinti studied ophthalmology wards air for fungal bioaerosol evaluation. He found that Penicillium spp., Aspergillus spp., Mucor and Alternaria were the most frequent fungi isolates, respectively. His quantitative results were close to that of thepresent study.
According to Table 2, the highest overall bioaerosol density was obtained for Shahid Beheshti and Bessat hospitals, respectively. These two hospitals have specialized services and they are the main hospitals of Hamedan, thus their high bioaerosol density may be because a large quantity of patients are referred to these hospitals. In addition, the location of these two hospitals is around the city (with opposite latitudes, geographically). Therefore, these hospitals’ indoor air quality may be affected by the outdoor air quality (21). Field observations showed that Shahid Beheshti hospital had no central and standard ventilation system for purification of hospital wards indoor air (Table 3). To supply indoor air, Shahid Beheshti hospital wards, use natural ventilation without pretreatment. This means that external air flows inwards through opened windows because of pressure or temperature differences. Also, some of the bioaerosol density may be because the hospital is located next to a green area.
Some researchers have addressed the impact of outdoor air on indoor air of closed places such as hospitals (11, 22). Although the Bessat Hospital had standard central ventilation, yet the existing system does not operate probably. For more detailed information, the system should be reviewed in terms of ventilation system design and operation. Some patient activities such as talking, walking in wards, sneezing and coughing cause an increase in emission and bioaerosol density of hospitals ward air (22, 23). Okhunoya et al. (23) concluded that patients and their activities might be factors affecting the concentration of bioaerosl density of indoor air.
Measurements of physical parameters such as airflow rate, showed that airflow was generally lower than 2fts-1 for all studied wards. In most wards airflow rate was zero or near to zero. Poor air flow rate does not allow movement, emission and dispersal of microorganisms from their resources. One way to prevent the entry of airborne pathogens is to control the positive inside air pressure. As airflow rate was zero at most hospital wards, thus there was no positive pressure. This results in the entry of airborne pathogens from the outside. Washing and disinfecting of ward floor, walls and some of the equipment can increase humidity therefore facilitating growth and survival of microorganisms. However, there was no disinfectant mechanism for indoor air of wards (Table 3), only for the operation room. Mean bioaerosol density of operation room’s air was more than 30 cfu/m 3 in a study performed by Choobineh et al. ( 7 ) and Jabari et al. ( 15 ). They concluded that ventilation defects, as well as unsuitable disinfection were the main cause of high bioaerosol density of surveyed wards.
As the results show, bioaerosol density of some hospital wards was more than 30cfu/m3. Most studied hospitals did not have air treatment systems. Therefore, should be taking measures to improve design and equipment installation. Finally, it is suggested that hospital managers attempt to qualitatively and quantitatively evaluate indoor air of hospitals periodically, and they should place and use air purification equipment in hospitals during the building stage.