1. Background
The Chlamydia trachomatis, is an obligate Gram negative intracellular bacterial parasite. It is one of the most prevalent sexually-transmitted pathogens inducing permanent complications in infected subjects, worldwide. The developmental cycle of these bacteria consists of two forms as follow: A. Elementary Body (EB), that is the extracellular infectious form and metabolically inactive; B. Reticulate Body (RB), the intracellular metabolically active form dividing by binary fission. C. trachomatis is involved in asymptomatic genital tract infections in both women and men (1, 2). C. trachomatis infection causes more severe complications in females than males (1). This infection leads to different diseases varying from acute and chronic pelvic inflammatory diseases, cervicitis, urethritis, endometritis, infertility and ectopic pregnancy (3), to different urogenital infections such as urethritis, prostatitis, and epididymitis (4). To date, the effect of these bacteria on male fertility is yet to be clarified. Several immunological factors including, antibodies (Abs), cytokines and chemokines have been reported to be involved in male infertility by several researchers (5, 6). Elevated levels of these factors in semen of men infected with C. trachomatis offer a role in immune defense of the male genital tract (7).
Previous reports (an ectocervical and endocervical cells, human urethral epithelial cells, and immortalized normal adult male prostate epithelial cells) demonstrated that chemokines play important roles in immune responses against this bacterium (5, 8). Chemokines are defined as a homologous group of 8-10 kDa peptides. They are further divided into four various subgroups including CXC, CC, C, and CX3C based on the position of cystein molecules in their biochemical configuration (9). All members of chemokine family, including CXC signal through specific seven transmembrane-G protein coupled receptors (CXC chemokine receptors (CXCRs)) (10).
According to the presence or absence of N-terminus conserved motif identified as ELR (containing glutamate, leucine, arginine) CXC chemokines themselves are further subdivided into two subclasses as ELR+ and ELR-(6, 11). CXCL1 and CXCL12 fit in ELR positive CXC chemokines while CXCL9 and CXCL10 are ELR-negative (12, 13). ELR-positive (CXCL12 and CXCL1) and ELR-(CXCL9 and CXCL10) CXC chemokines are neutrophils and T lymphocytes recruiters, respectively. Therefore, increased expressions of these chemokines in response to C. trachomatis result in inflammation in the infected tissues. Activation of these chemokines is frequent in autoimmune and inflammatory disorders (14). Accordingly, previous studies demonstrated that immune, including chemokines, responses against C. trachomatis determine the outcome of infection (15). Additionally, during infection with C. trachomatis, an inflammation occurs due to the predominant roles of these chemokines in recruitment and activation of lymphocytes.
2. Objectives
The current study aimed to evaluate the relationship between the seminal levels of CXCL1, CXCL9, CXCL10 and CXCL12 in patients with C. trachomatis infection.
3. Materials and Methods
3.1. Collection of Semen Samples
During this cross-sectional study, 585 consecutive men attending at the Avesina Research Institute, Tehran-Iran, were selected for the study. Ethical Committee for Researches in Rafsanjan University of Medical Sciences approved using semen samples for the analysis. All of the participants including patients with C. trachomatis infection and the healthy controls were free of sexually transmitted diseases and had not received chemotherapy or radiotherapy treatment. None of them had the background of vasectomy, and abnormally low semen volume; a retrograde ejaculation and hypogonadism were considered as excluding criteria. All of the participants underwent semen analysis for infertility investigations. They were asked to avoid sexual intercourse for two to five days before attending the examination. The healthy controls were age/sex matched with the C. trachomatis infected patients. All of the semen samples were collected into standard containers according to the methods outlined in World Health Organization (WHO) laboratory manual (16). Semen samples were stored at -20ºC for further studies.
3.2. DNA Extraction
A DNA extraction Kit (Bioneer, South Korea) was used for semen samples according to the manufacturer’s guidelines. The extracted DNA was checked using β-actin primers to examine either the presence of PCR inhibitors or DNA quality, then the resultant DNA was stored at -20°C until using in PCR analysis.
3.3. PCR Condition
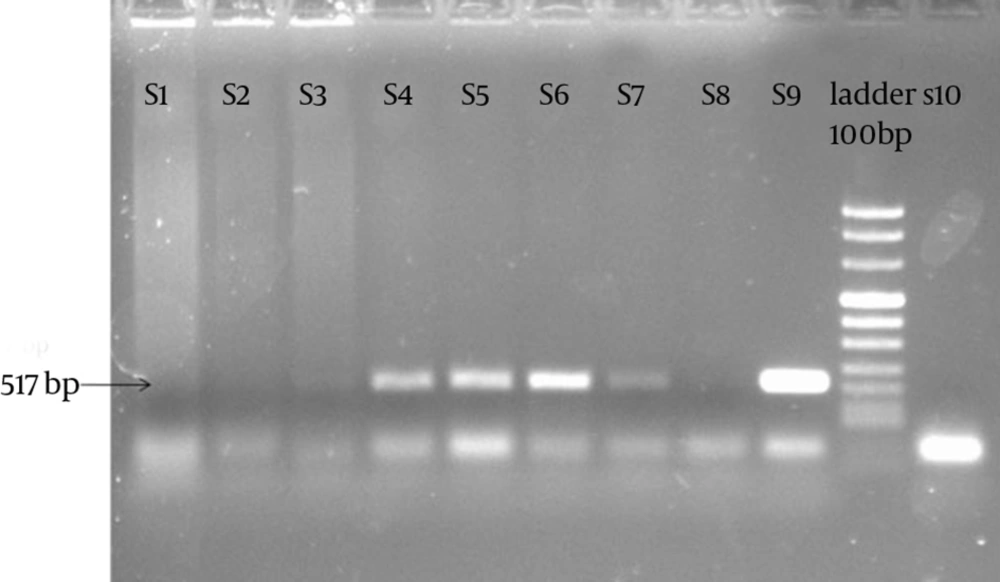
PCR was performed by forward (5'-GGACAAATCGTATCTCGG-3') and reverse (5'-GAAACCAACTCTACGCTG-3') primers (Cinaclon, Iran) specific for K/SotonK1 plasmid pSotonK1 gene of 517 base pair (bp) C. trachomatis to diagnose the infection. The following program was used for PCR amplification: 35 cycles of 93ºC for 60 seconds, 60ºC for 20 seconds, and 72ºC for 40 seconds and C. trachomatis genome strain K1 was used as positive control.
3.4. Chemokine Assays on Semen Samples
Concentrations of CXCL1, CXCL9, CXCL10 and CXCL12 in semen were measured by ELISA (R&D Systems, Minneapolis, UK). The sensitivity of kits was 2 pg/ mL and inter and intra-assay assessments of reliability of the kits were conducted and data were only used when inter and intra assays produced scores of CV < 14% and CV < 3%, respectively.
3.5. Statistical Analysis
Results of the current article were presented as mean ± SEM (standard error of mean) for numeric variables, and absolute frequencies and percentages for categorical variables. Student’s t-test was used for analysis of continuous variables including CXCL1, CXCL9, CXCL10 and CXCL12 and the normality of the distribution of the data was examined by applying the Kolmogorov-Smirnov test. In order to perform the statistical analysis, SPSS software version 18.0 (SPSS Inc. Chicago, IL) was employed. All P Values were two-tailed, with statistical significance defined as P ≤ 0.05.
4. Results
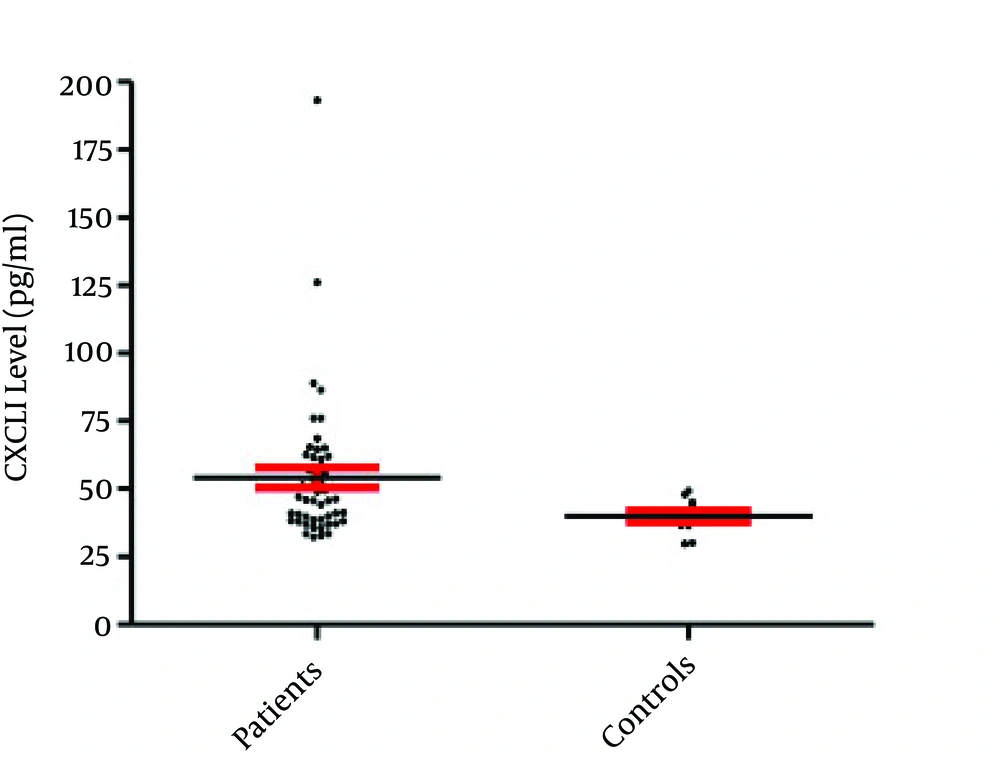
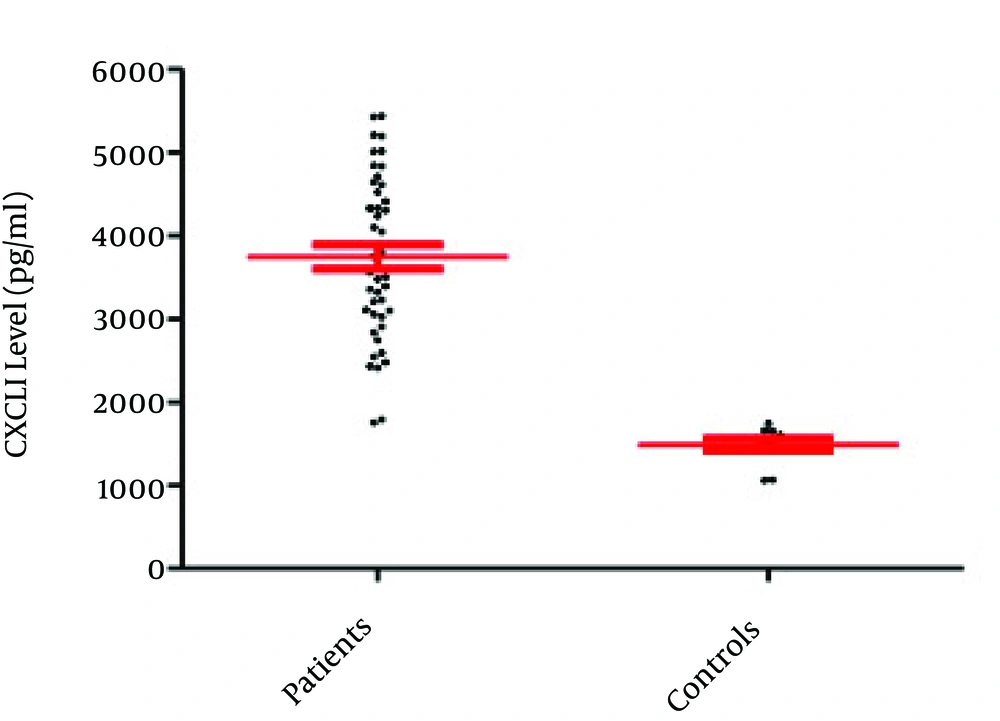
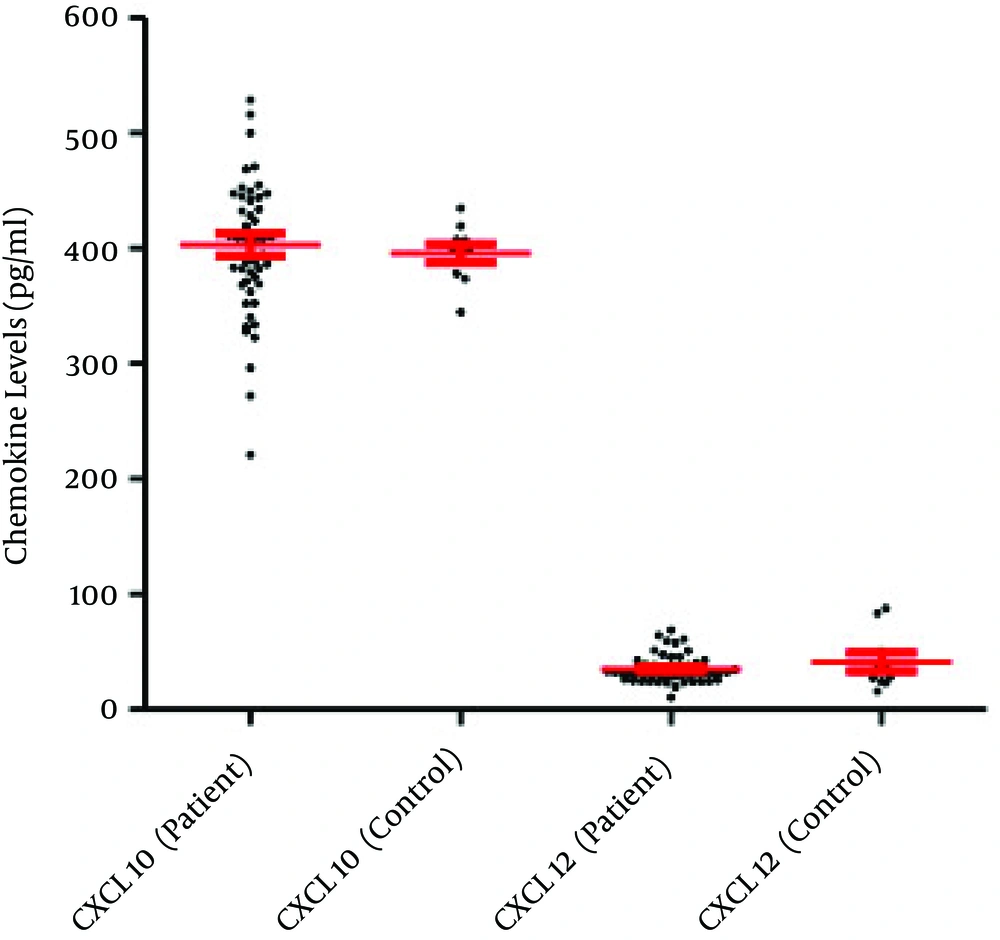
The current study results indicated that only 50 out of 585 semen samples (8.55%) were infected with C. trachomatis (based on the PCR results) (Figure 1). The obtained results demonstrated that the mean semen levels of CXCL1 in the patients and controls were 54.13 ± 3.76 pg/mL and 39.91 ± 2.17 pg/mL, respectively (P = 0.027) (Figure 2). It was observed that the seminal CXCL9 levels were 3742.71 ± 145.75 pg/mL and 1488 ± 75.27 pg/mL in the patients and controls, respectively (P < 0.001) (Figure 3). The CXCL10 levels were 403.18 pg/mL ± 9.57 pg/mL and 395.67 ± 8.02 pg/mL in the patients and controls, respectively (P = 0.729). Finally it was also found that the seminal CXCL12 levels were 35.07 ± 1.73 pg/mL and 41.21 ± 7.96 pg/mL in the patients and controls, respectively (p= 0.470) (Figure 4).
Overall, results of the present investigation revealed that the levels of CXCL1 and CXCL9 were significantly different from those of the controls and there was a positive correlation between chemokines and infection. It was also observed that although CXCL10 followed the pattern of these inflammatory (CXCL1 and CXCL9) chemokines, its elevated level was not significant. Ultimately, sustained seminal levels of CXCL12 in patients and controls were observed.
5. Discussion
In the present study, the semen levels of CXCL1, CXCL9, CXCL10 and CXCL12 were investigated in patients with C. trachomatis infection and healthy controls. Notably, semen levels of both CXCL1 and CXCL9 significantly increased in patients, which may be possibly related to their functions as pro-inflammatory. Chemokines are involved in the processes of neutrophil and monocyte lineage infiltration into the infected testis. It is well established that CXCL1 acts as migratory factor for neutrophils while CXCL9 is a lymphocyterecruiter. It has also been well evidenced that the innate immune responses of epithelial cells and urogenital tract resident immune cells are the first line of defense against C. trachomatis (17). Furthermore, chemokines and cytokines are produced during the innate immune responses of the chemoattract inflammatory cells against the infected tissues to control infection.
Chemokines also initiate adaptive immune response against the bacteria and further clearance processes (18). Therefore, based on the current study results, it may be concluded that both CXCL1 and CXCL9 are critical chemokines involved in the innate immune responses against C. trachomatis infection. Previous studies demonstrated that CXCL1 is produced by several immune and non-immune tissue/cell systems, including macrophages, neutrophils and epithelial cells with predominant neutrophil chemoattraction activities (19). Moreover, it has also been documented that CXCL9 play important roles in infiltrating of the activated T lymphocytes towards the infected and inflamed tissues (20). Thus, CXCL1 and CXCL9 may regulate activation and infiltration of neutrophils and T lymphocytes to the infected regions allowing progression of immune responses against C. trachomatis. Although, several research teams reported the presence of pro-inflammatory cytokines in the semen of patients infected with C. trachomatis, to best of the authors’ knowledge, the present study is the first to address elevated levels of CXCL1 and CXCL9 in semen of these patients. The other member of IFN-γ inducible inflammatory chemokines (CXCL10) also increased, which was not significant. Elevated levels of these chemokines as downstream targets of IFN-γ could be presumably related to their function as members of inflammation pathway rather than their other properties.
A previous study demonstrated that the expression of CXCL9 depends on IFN-γ and activation of its signaling pathway transcription factors, especially STAT1 (21). Additionally, the ability of the male urogenital tract epithelial cells to produce IFN-γ has been documented and this cytokine is present in seminal fluid (22). Studies revealed that both CXCL1 and CXCL9 are inducible by IFN-γ and other pro-inflammatory cytokines such as TNF-α (13). It suggests that production of IFN-γ in response to bacterial infections, including C. trachomatis, may induce the expression of CXCL9. In agreement with the results of the current study, several other research teams reported that subsequent to infection with C. trachomatis, epithelial cells produce a wide variety of pro-inflammatory mediators, including CXCL1, CXCL8, CXCL16, granulocyte/monocyte-colony stimulating factor (GM-CSF), IL-1, IL-6, and TNFα (23). Mackern Oberti et al. have examined the prostate epithelial/stromal cells dependent immune responses to C. trachomatis and observed that it could infect murine prostate cells by the development of large inclusions. Moreover, their results demonstrated that prostate cells have responded to C. trachomatis infection by production of pro-inflammatory chemokines inducing CCL2, CXCL1, and CXCL2 (19). They also observed that the chemokine productions by prostate cells were performed via activation with toll-like receptor, pathogen-associated molecular patterns interaction (19).
Finally, according to the findings of the current study and the other aforementioned studies, it may be concluded that several men genital system cell types such as prostate, epithelial and resident immune cells are involved in the development of immunological responses against C. trachomatis and it might be mediated by CXCL1 and CXCL9 but not CXCL10 and CXCL12. Based on these findings, these chemokines could be considered as future targets for immunotherapy to eradicate the C. trachomatis infection and its complications including infertility.