1. Background
Rabies is a major zoonotic viral disease which is regarded as a health problem around the world (1). In Asia, eight countries have been reported as rabies free, including Japan, Malaysia, Hong Kong, Singapore, Taiwan, Qatar, Bahrain, and the United Arab Emirates (2). This disease is caused by a group of neurotropic viruses of the family Rhabdoviridae, order Mononegavirales, genus Lyssavirus (1). According to cross protection tests and molecular biological analysis, the genus Lyssavirus is classified into seven distinct genetic lineages: 1) the classical rabies virus (RABV), 2) Lagos bat virus (LBV), 3) Mokola virus (MOKV), 4) Duvenhage virus (DUUV), European bat Lyssavirus type 1 (EBLV-1), European bat Lyssavirus type 2 (EBLV-2), and the Australian bat Lyssavirus (ABLV) (3). Recently discovered Lyssaviruses, Aravan (ARAV), Khujand (KHUV), Irkut (IRKV) and West Caucasian bat virus (WCBV) have also been introduced (4).
The rabies virus includes five structural proteins, a nucleoprotein (N), a phosphoprotein (P), a RNA polymerase (L), matrix protein (M) and glycoprotein (G). The N, P and L proteins together constitute the nucleocapsid (NC) (5). Prevention and control of rabies in human and animals (especially in the wild life), is based on the use of rapid and specific diagnostic techniques and is established according to clinical, epizootiological and laboratory assessments (6). Rabies should still be considered as an important zoonotic health problem in Iran. However, currently the disease is being controlled if we compare the situation with 40 years ago (2, 7). Although official reports claimed that the principal maintenance host is domestic dogs, an investigation by the Pasteur Institute of Iran (collaborating with the world health organization for Reference and Research on Rabies) revealed that wolves are responsible for rabies transmission to humans (7, 8).
The world health organization (WHO) and the International Office of Epizootics (OIE) recommend the fluorescent antibody technique (FAT), which is done on specimens from central nervous system, skin biopsy and salivary glands as the preferable laboratory method and gold standard for diagnosis of rabies (6, 9, 10). The nucleoprotein (NP) with an approximate molecular weight of 50 KDa, consists of 450 amino acids, and it is the most preserved antigenic protein for different rabies strains (1, 5). It is the main component of the rabies virion that contains group-specific antigenic determinants, which are important in diagnosis of rabies infection (1, 11).
2. Objectives
This study was conducted to extract NP from Baby Hamster Kidney cell clone (BSR) infected with the Pasteur vaccine strain (PV) and to purify this NP in order for it to be used in the future for producing a fluorescent anti-NP conjugate with high sensitivity and specificity, which can be used in FAT.
3. Materials and Methods
3.1. Virus Strain and Cells
The virus strain used in this study was the PV strain that is the standard rabies virus vaccine strain; the virus was propagated in BSR cells in the presence of Dulbecco modified Eagle’s medium (DMEM) (Sigma-Aldrich, USA) containing 10% fetal bovine serum (FBS) (Sigma-Aldrich, USA).
3.2. Titration of the Pasteur Vaccine Strain
Viral titration was carried out on BSR cells according to the WHO instructions (12, 13). Serial tenfold dilutions of the seed virus were prepared. Incubation was performed in an open system for 48 hours at 37°C with 5% carbon dioxide (CO2). Viral infection was detected by FAT (14, 15). The final titer of the virus was calculated according to the following formula:
Titer = [average number of fluorescent foci in the last dilution where fluorescence is still visible] [dilution × 3] [20, 000] (12, 16).
3.3. Extraction of Nucleoprotein
Viral particles’ propagation was done in 30 cell culture bottles of 150 cm2. Two milliliters of 106/mL BSR cells were added to each bottle and incubated at 37°C until a monolayer was formed. After three days, the medium was poured out and the monolayer of the cells was washed with 20 mL phosphate buffered saline (PBS) (Sigma-Aldrich, USA) solution at pH 7.4. Then, 9 mL of PV suspension was inoculated on to each monolayer and incubated for 1 hour at 37°C with 5% CO2 and the content of the bottles were gently stirred every 10-15 minutes to increase virus adsorption. After virus adsorption, the cell monolayers were washed with 20 mL PBS by adding Dulbecco's modified Eagle's medium(DMEM) containing 10% FBS; the volume was adjusted to 50 mL.
The battles were incubated for 24 hours at 37°C with 5% CO2 and then for 48 hours at 34°C with 5% CO2 and subsequently, the supernatant was discarded and the monolayer was washed with 20 mL of PBS. The cells were collected using a cell scraper with a blade length of 20 mm (MIDSCI, TPP, USA) and were suspended in ice-cold 0.5 M sodium chloride/Tris-HCl (NT) buffer at pH 7 and centrifuged twice at a speed of 900 g and 4ºC for 10 minutes (16, 17). Cell lysis was carried out by adding 5 mL of ice-cold, sterile, deionized water containing 25 µL of aprotinin per milliliter (18). This mixture was incubated for 1 hour at 4°C and clarified by centrifugation at a speed of 1,000 g and 4°C for 20 minutes. The supernatant was collected and this procedure was repeated twice. The supernatants from each round were combined and clarified by centrifugation at a speed of 12,000 g and 4°C for 10 minutes and finally, the supernatant was separated.
3.4. Purification of Nucleoprotein
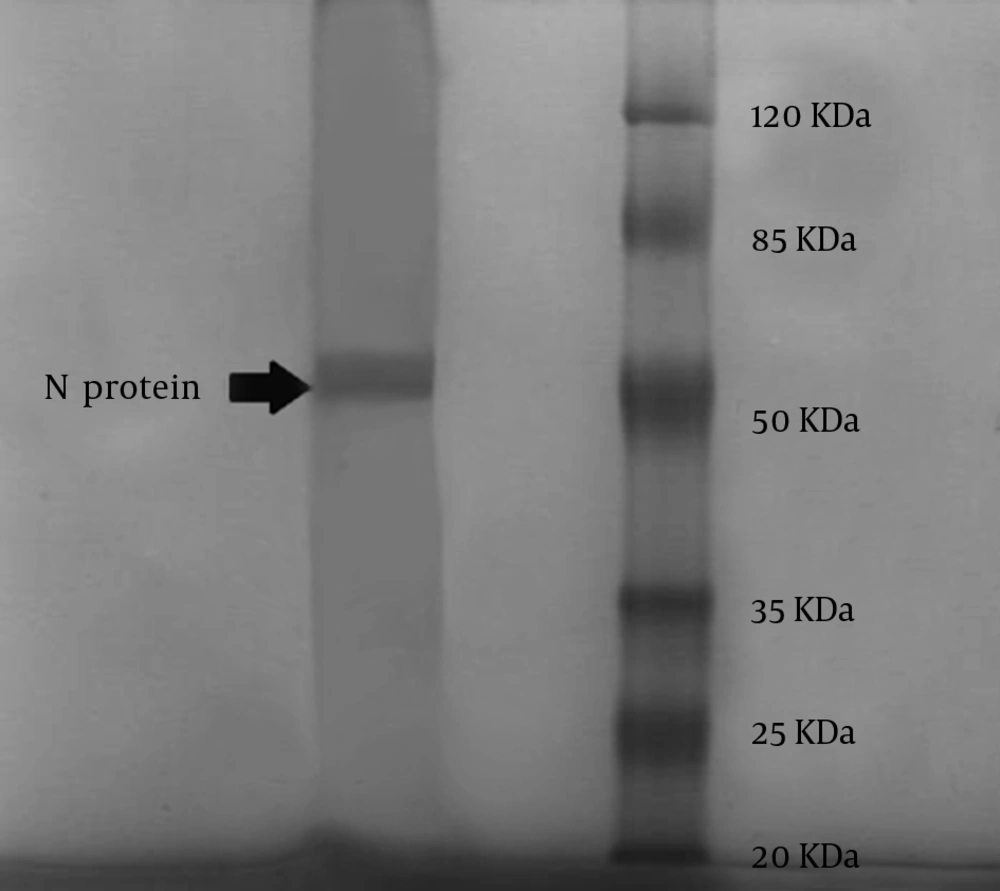
According to the WHO instructions, rabies virus NP was purified by ultracentrifugation (Beckman type 90Ti, USA) in CsCl gradient (16). For each 3 mL of supernatant of NP, 2 g of CsCl was added to 5 mL polycarbonate tubes (Beckman type 90Ti, USA). The final volume was adjusted to 5 mL by adding NT buffer at pH 7.6 and centrifuged at speed of 50,000 rpm for 10 hours at 4°C. The band (approximately 0.5 mL) was collected through the top of the tube using a 23-gauge needle (Reno, Nevada, USA). The fraction was dialyzed against pH 7.6 NT buffer for 24 hours at 4°C. Then, the protein concentration was measured by a spectrophotometer at a wave length of 280 nm. In order to confirm NP presence, 5 µL of the sample of purified RNP was loaded on a 12.5% SDS-PAGE gel, with 5% stacking and stained with Coomassie blue (Merck, Germany) (10, 15). We also performed a modified Western blot analysis using only the Bio-Rad conjugate (Bio-Rad, France) to stain a fixed blot of the extracted NP, as this conjugate is a specific antibody against the Rabies NP.
4. Results
Several serial passages were done to obtain the 105 focus-forming dose (FFD) titer of the virus. The volume of lysate was 15 mL and after purification; it became 2.5 mL, with a concentration of 3.25 mg/mL. The extracted rabies virus NP from infected BSR cells was at the same molecular weight (50 KDa) as Rabies virus NP by SDS/PAGE. The obtained clear band with an approximate molecular weight of 50 KDa is depicted in Figure 1. There were also some non-specific bands on SDS-PAGE, which usually form after purification of intra cellular proteins such as viral proteins with no interference with the specific NP band. The NP band was confirmed again by Western blot analysis.
5. Discussion
Rabies virus causes the formation of specific aggregates of viral material, which are called Negri bodies (5,616). These structures are found in the cytoplasm of infected neurons or in cell cultures and are considered as the most important factor for confirmation of rabies virus infection. Negri bodies are usually between 0.24-27.0 µm in size and 2-10 µm in diameter (5). The most prominent characteristic of Negri bodies is their internal structure. Electron microscopy observations showed that Negri bodies are made-up of a matrix of filamentous material comprised of viral NP (19). Conserved antigenic sites on the NP result in identification of all rabies virus strains by using anti-rabies antibody conjugates (3).
At present, the method of choice for detection of these cytoplasmic structures is FAT (6). Fluorescent antibody technique is one of the most rapid techniques for rabies antigen identification in the brain and salivary glands of human and animals suspected of infection by the rabies virus (12, 15). Using a good quality conjugated antiserum should be considered as one of the main requirements for precise diagnosis of rabies infection (17). The FAT has approximately 100% specificity and sensitivity if a proper conjugate is used (6). In 1973 Dean and Abelseth, used inactivated rabies infected mice brain suspensions as a source of viral antigens for immunization of animals for obtaining concentrated specific antibodies (12). In 1974 Atanasiu and his colleagues reported the first production of high-titer conjugates using rabies virus NC (13, 14).
Since FAT by using the anti-NP conjugate is much more sensitive than using conjugates specific to the total virion, we attempted to obtain this protein in its purified form, in order to produce the conjugate in the future (6, 12). In this study the virus was obtained in a similar titer as previously described by a Brazilian study (10). The extracted rabies virus NP was confirmed by SDS-PAGE and Western blotting as the viral strain used in this project was the standard rabies virus strain (PV). There was also some partial purification of NP, which resulted in a few minor bands on SDS-PAGE after purification with no probability of interference with the specific conjugate (10, 12). Commercial conjugates are against the total virion or specific to the rabies NP (such as the conjugate from Bio-Rad, France), or they are prepared from monoclonal antibodies (3). Rabies virus is amongst health problems in Iran (2, 20).
In 2006, more than 130,000 people received post-exposure prophylaxis thus, a sensitive surveillance system is necessary to detect rabies infections using the most accurate laboratory methods recommended by WHO (12, 15). At the Pasteur Institute of Iran (WHO Collaborating Center for Reference and Research on Rabies), a rabies conjugate is provided by Bio-Rad with a high price. Recently, there has been increasing problems in purchasing commercial conjugates from foreign companies. In addition to cost, there are other problems such as delays in delivery, changes in quality and affinity of conjugate and sometimes lack of access. Here, we tried to overcome these problems and we successfully achieved the first step for producing the anti-NP conjugate by extraction and purification of rabies virus NP from cell culture.