1. Introduction
Acinetobacter baumannii has become an important pathogen that causes nosocomial infections such as bloodstream and wound infections, ventilator-associated pneumonia and meningitis (1). Approximately 10% of Gram-negative and 4% of nosocomial meningitis occur as a result of A. baumannii infection (2). Neurosurgical patients with cerebrospinal fluid (CSF) leakage have a high risk of acquiring meningitis. A. baumannii is a serious pathogen that causes meningitis in these patients (3) and many authors worldwide have reported post neurosurgical meningitis due to A. baumannii (4). Since A. baumannii strains are multidrug resistant, nosocomial infections associated with neurosurgical procedures such as nosocomial meningitis caused by A. baumannii had a high rate of mortality (5, 6); two studies have reported a mortality rate ranging from 71.4% to 72.7%, respectively for A. baumannii meningitis in neurosurgical patients (5, 7).
In the recent years, as a result of excessive use of antibiotics, the epidemiology of post-neurosurgical meningitis may have changed, and because treatment options for multidrug resistant A. baumannii are limited, the rate of meningitis due to this bacterium will be increased (2). Colistin is the last resort for treatment of pan drug-resistant A. baumannii, but increased use of this antibiotic has led to progress of colistin-resistant strains (1). The first colistin heteroresistance in A. baumannii was described in 2006. This type of resistance may be the primary stage that upon contact with colistin, resistant subpopulations can proliferate (8). Colistin heteroresistance seriously warns that colistin should be used properly. If colistin is used improperly, resistance may develop and lead to treatment failure (9). We report a case of post-neurosurgical meningitis due to colistin heteroresistant A. baumannii, with some changes in antibiotic susceptibility profile during intratreatment with colistin.
2. Case Presentation
Our case was a 20-year-old man who had a gunshot trauma to the abdomen, which had exited from his back. The patient was admitted and underwent laparotomy three times. There was a debrided ulcer on his back with leakage of CSF. One week after his discharge, the patient became febrile (T ≥ 39°C) and was admitted to our unit with a diagnosis of meningitis and treated with meropenem, vancomycin and amikacine. Cerebrospinal fluid analysis was as follows: total cell count = 230, total white blood cells (WBC) = 200, polymorph nuclear (PMN) = 70%, lymphocytes (LYM) = 30%, glucose 39 mg/dl and protein 40 mg/dL. The radiological study revealed hydrocephalus, therefore extra ventricular drainage (EVD) was implanted. After four days, samples from the ulcer, urine and CSF yielded A. baumannii. Finally the patient was referred to our center (Golestan Hospital, Ahvaz, southwest of Iran). At this center, EVD was revised twice and the patient received antibiotic therapy with ampicillin/sulbactam, piperacillin/tazobactam, rifampin and ciprofloxacin in different combinations.
On the day of the admission a CSF sample was taken and cultured. After growth, colistin sensitive A. baumannii (isolate number 1) was recovered. After four days, colistin (intravenous and intrathecal) was initiated. Five days after administration of colistin, another CSF sample was taken and cultured again, and colistin resistant A. baumannii (isolate number 2) was recovered. The patient was still ill and did not improve. On the thirteenth day of treatment with colistin, a CSF sample was taken again, analyzed and cultured. However, the culture was negative for A. baumannii but CSF analysis showed meningitis pattern that was as follows: total cell count = 11600, total white blood cells (WBC) = 1600, polymorph nuclear (PMN) = 85%, Lymphocytes (LYM) = 15%, glucose 25 mg/dL and protein 65 mg/dL. Colistin treatment was continued until the last day of his life. The patients expired after 26 days of hospitalization. According to the importance of diagnosis and treatment of A. baumannii meningitis, and since this case of meningitis was particular and rare, we decided to do some additional experiments on two isolates of the A. baumannii.
Conventional microbiology tests were performed for initial identification of A. baumannii (10). DNA was extracted by sodium dodecyl sulfate (SDS) and the proteinase K method (11) and, blaOXA-51-like gene and partial sequencing of rpoB gene were performed by specific primers (12, 13). A. baumannii NCTC 12156 (ATCC 19606) was used as the positive control (14). Antimicrobial susceptibility testing of the two isolates to the following antibiotics was evaluated by the disk diffusion method (15). The tested antibiotics were as follow: imipenem 10 µg, meropenem 10 µg, ceftazidime 30 µg, cefepime 30 µg, ceftriaxone 30 µg, colistin 10 µg, piperacillin 100 µg, piperacillin-tazobactam 100/10 µg, polymyxin B 300 unit, gentamicin 10 µg, tobramycin 10 µg, amikacin 30 µg, tetracycline 30 µg, ciprofloxacin 5 µg, trimethoprim-sulfamethoxazole 1.25/23.75 µg, rifampin 5 µg, aztreonam 30 µg and ampicillin-sulbactam (10/10 µg) (MAST, Group Ltd, Merseyside, UK). Minimum inhibitory concentration (MIC) of colistin, imipenem, meropenem and tigecycline were determined by the E-test (Liofilchem, Italy). The results of disk diffusion and E-test were interpreted according to CLSI guidelines. The United States Food and Drug Administration-approved criteria for Enterobacteriaceae was used for the tigecycline breakpoint (16).
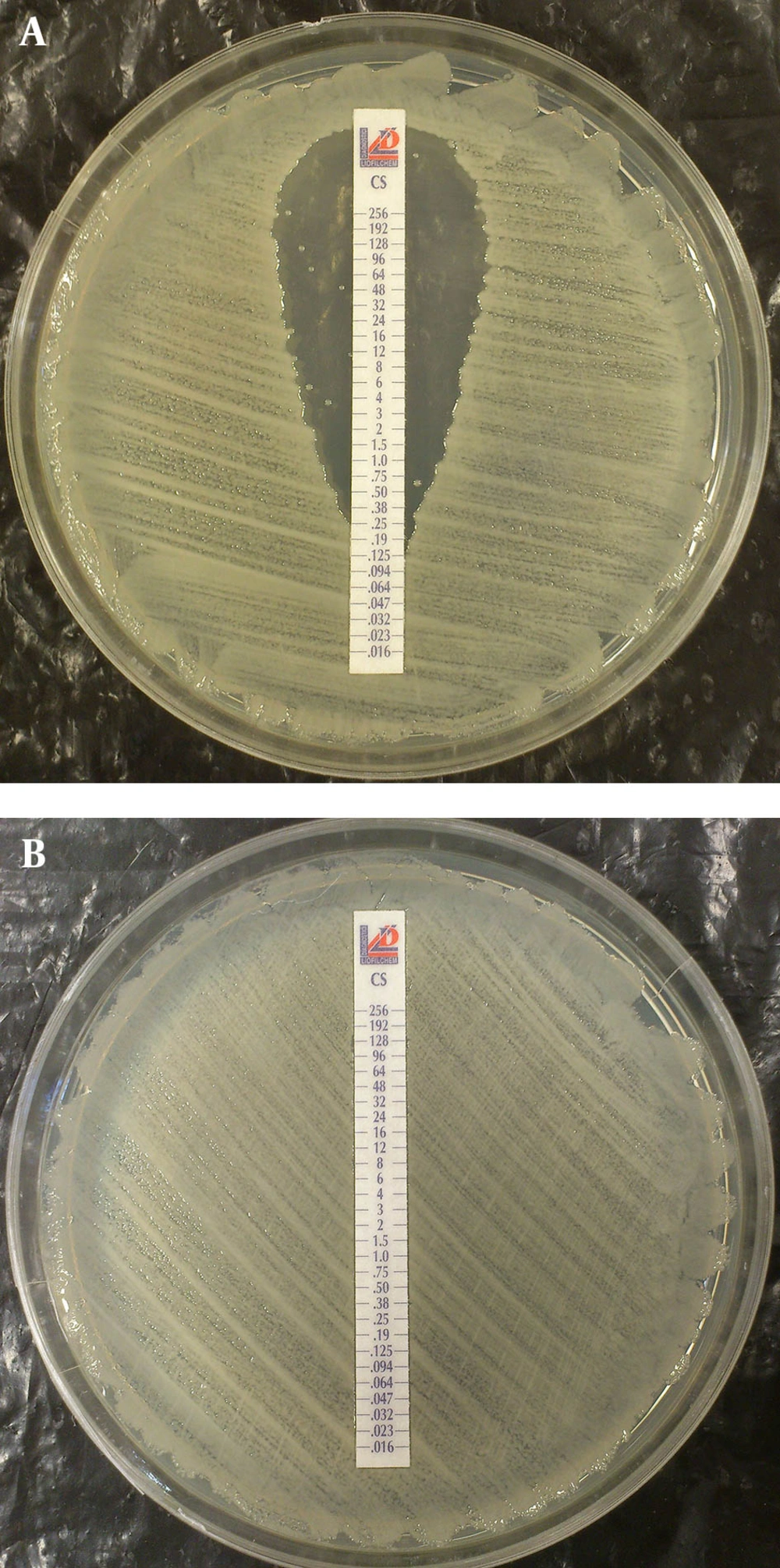
Multiplex PCR for detection of oxacillinase genes such as: blaOXA23-like, blaOXA-24-like and blaOXA-58-like were performed as previously described (14). Metallo ß-lactamase production and encoding genes such as: blaIMP, blaVIM, blaSPM and blaNDM were investigated by PCR (17, 18). Both isolates were examined by REP-PCR with primers according to the Bou et al. protocol with some modifications (11). According to the PCR results, both isolates were blaOXA-51-like positive and according to sequences of rpoB gene alignment, both isolates were confirmed as A. baumannii. Isolate 1 was resistant to all of tested antimicrobial disks except polymyxin B, colistin and ampicillin-sulbactam. Results of E-test showed that isolate 1 was resistant to imipenem and meropenem with MIC > 32 µg/mL and 12 µg/mL, respectively, yet was sensitive to colistin with MIC 0.25 µg/mL. Although disk diffusion results showed that isolate 1 was resistant to tigecycline yet the E-test result revealed tigecycline MIC 1.5 µg/mL, and isolate 1 was categorized as sensitive to tigecycline according to the E-test result. However isolate 1 was sensitive to colistin, but within the colistin zone of inhibition, eight colonies grew, which are indicated in Figure 1-A.
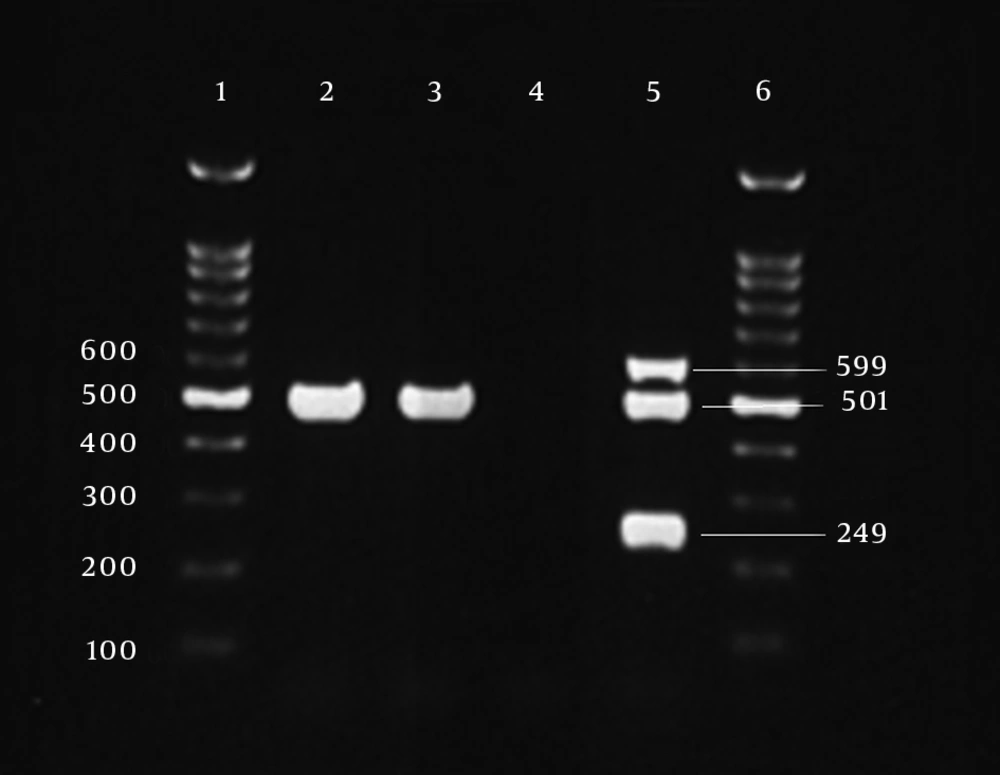
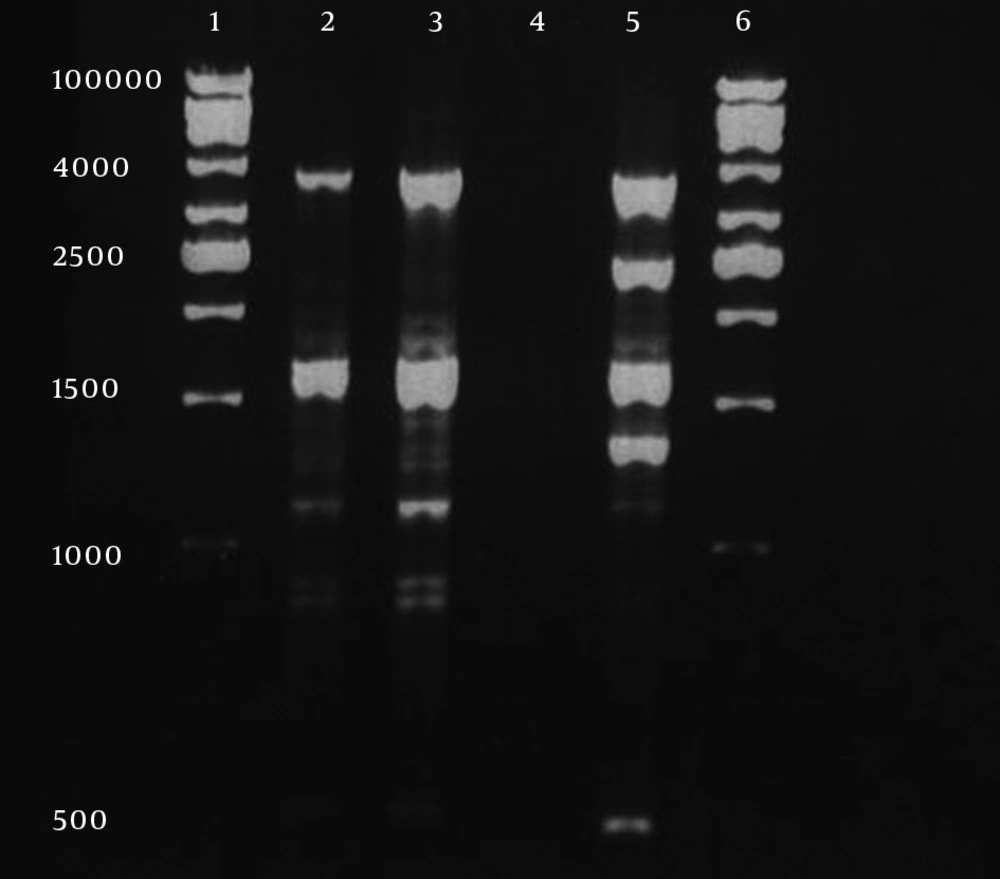
Antibiogram results of the second isolate that was recovered after colistin therapy was similar to the first isolate but some changes had occurred. The second isolate was completely resistant to colistin with MIC 256 µg/mL (Figure 1B). By disk diffusion, isolate 2 was resistant to both of polymyxin B and colistin but remained sensitive to ampicillin-sulbactam. The MIC results for imipenem, meropenem and tigecycline were unchanged for the second isolate. Multiplex PCR revealed that two isolates had the blaOXA23-like gene (Figure 2). Other investigated genes were negative for both isolates. By phenotypic tests, metallo-ß-lactamase production was negative for the two isolates. Results of REP-PCR revealed that the two isolates were derived from a single strain and were same (Figure 3).
3. Discussion
Nosocomial meningitis due to A. baumannii is fatal and its treatment is difficult (19). Recently, post neurosurgical meningitis caused by colistin heteroresistant A. baumannii has been reported (20, 21). Due to difficulties in detection, the prevalence of colistin heteroresistance is not yet well understood and is not routinely used in most laboratories (9, 21) but Overall, the colistin heteroresistance rate in A. baumannii was much higher than the resistance rate (9). In the present study at the beginning of colistin treatment, isolate 1 was susceptible to colistin with MIC 0.25 µg/mL but some colonies were grown within the zone of inhibition that indicate heteroresistant subpopulations were present. Five days after treatment with colistin, isolate 2 was recovered with colistin MIC > 256 µg/mL that was completely resistant to colistin. It seems that during intra-treatment in vivo, a colistin resistant subpopulation was selected. One similar finding was reported by Rodriguez et al. from Argentina (21).
The results of our study is in contrast to that of Rodriguez et al. because in our study we were not able to treat meningitis but Rodriguez et al. successfully treated the meningitis due to A. baumannii. The difference between the results of the two studies could be due to the susceptibility pattern of the two isolates to rifampin, because our isolates were resistant to rifampin but in the Rodriguez et al. study the A. baumannii isolates were susceptible and responded to rifampin and colistin therapy. Moreover, successful treatment of post-neurosurgical meningitis caused by multidrug-resistant A. baumannii with intrathecal colistin has been previously reported (2) but unfortunately in this study, colistin was not effective even through intrathecal use, because the first isolate was heteroresistant and became completely resistant during the treatment. However, previous exposure to colistin may be a risk factor for development of heteroresistance (9) yet in this patient, no treatment with colistin was performed previously.
In vitro synergy between rifampin and colistin has been shown previously (20). Rifampin has an excellent CSF penetration and was used for the treatment of meningitis caused by A. baumannii (2, 22). Unfortunately, our isolates in addition to colistin were resistant to rifampin thus, although we used a combination of two drugs (colistin and rifampin) treatment was not effective. The two isolates were sensitive to ampicillin-sulbactam in vitro and we used this antibiotic for treatment, but the patient did not respond to this antibiotic either. Tigecycline has in vitro activity on pan-drug resistant acinetobacter and some studies reported successful treatment of meningitis caused by multidrug resistant A. baumannii with tigecycline, yet this treatment option is very expensive and is not easily available in developing countries (23, 24). Moreover, our isolates were susceptible to tigecycline in vitro, but tigecycline is not available in Iran. Interestingly we observed a strange phenomenon regarding polymyxin B. The first isolate of A. baumannii was susceptible to polymyxin B by disk diffusion, but the second isolate was completely resistant to polymyxin B without any previous exposure to this antibiotic, because polymyxin B was not use in the treatment of the patient. We could not find any reason for this phenomenon.
It is assumed that because colistin and polymyxin B belong to one family, cross-resistance may occur between them. In comparison to the first isolate, in the second isolate other antibiotics MIC, and disk diffusion profile, were not changed intratreatment and the second isolate remained sensitive to ampicillin-sulbactam and tigecycline in vitro. For detection of carbapenemase genes we investigated class D, OXA genes that are the most active for resistance to carbapenems in A. baumannii (25). We found that two isolates had blaOXA23-like gene. Other investigated genes were negative in both isolates. To confirm that both isolates were from identical sources and that the patient was not infected with other A. baumannii strains during his stay in the intensive care unit (ICU), REP-PCR was performed according to the Bou et al., protocol (11). Results of REP-PCR revealed that the two isolates were derived from a single strain. This case report is unique because generation of polymyxin resistant A. baumannii during colistin therapy has not been reported previously. In the present study, the disk diffusion method failed to detect colistin heteroresistance subpopulation in the first isolate and we recommend, in isolates that were recovered for the first time from CSF or were recovered during intratreatment, colistin MIC should be measured with E-test or other MIC methods.