1. Background
Toxoplasma gondii is a multi-host obligate intracellular protozoan parasite, causing zoonotic infections throughout the world. Definitive hosts for this coccidian parasite are felids (both domestic and wild); and the intermediate hosts are mammals and birds (1, 2). The felids disseminate oocysts into the environment, where they can infect all types of warm-blooded animals (wildlife, companion animals, domestic livestock), including humans. The intermediate hosts can be infected by ingesting food or water contaminated with oocysts, eating undercooked meat with tissue cysts or by transplacental infection with tachyzoites (2, 3).
Humans acquire toxoplasmosis mainly through ingestion of tissue cysts present in undercooked meat or by accidentally ingesting oocyst (4). Infections involving immunocompetent humans usually result in asymptomatic cases, although cervical lymphadenopathy or ocular diseases occur in some of them (up to 10%). Occurrence of the parasite in immunocom-promised hosts and infants can however be an important cause of morbidity and mortality, causing serious disease symptoms (5, 6). In immunodeficient individuals such as HIV patients, the parasites can cause severe toxoplasmic encephalitis (7, 8). Infections in infants can cause mental retardation, loss of vision and jaundice.
In Iran about 50% of the human population has been exposed to T. gondii, which makes toxoplasmosis one of the major public health problems (9). Contact and interaction between domesticated animals and humans are known to be responsible for an increased risk of transmission of the parasite (10).
2. Objectives
There is a need for regular updates on the prevalence rates of this zoonotic pathogen in different animal species in order to provide useful epidemiological data to plan control strategies and eventually stem the consequences associated with the pathogens. The current study aimed to determine the presence of T. gondii in the blood samples of cattle, camels and sheep in Isfahan and Chaharmahal va Bakhtiary provinces by Polymerase Chain Reaction (PCR) method. The obtained information through this sensitive technique would add value to the available data most of which rely on serology.
3. Materials and Methods
3.1. Study Area
The present study was conducted in Isfahan and Chaharmahal va Bakhtiary provinces, Central and South-West of Iran respectively. Livestock production in Isfahan province is mostly traditional whereas in Chaharmahal va Bakhtiary province commercial livestock production is common. Samples were collected from randomly selected cattle, camels and sheep from slaughterhouses and peripheral farms.
3.2. Study Design, Sample Size Determination and Animal Sampling
It was a cross-sectional study conducted from January to March 2013. Blood samples were collected from the jugular veins of 155, 122 and 95 randomly selected cattle, camels and sheep, respectively. Respective numbers of samples from the abattoirs and farms were 100 and 55 for cattle, 90 and 32 for camels; and 65 and 30 for sheep, respectively. Five milliliters of blood was collected from each animal, stored in heparinized vacutainer tubes and conveyed on ice to the laboratory for analysis.
3.3. DNA Extraction
Genomic DNA was extracted from blood specimens using CinnaGen DNA extraction kit (Cinnagen, Tehran, Iran) according to the manufacturer’s instructions. The extracted DNA was quantified by spectrophotometric measurement at a wavelength of 260 nm according to the method described by Sambrook and Russell (11). Extracted DNA samples were stored frozen at -20°C until used for molecular analysis using PCR at the Biotechnology Research Center of the Islamic Azad University of Shahrekord.
3.4. Gene Amplification
The PCR was performed on 50 μL total reaction volume including 5 μL of 10x PCR buffer [70 mM Tris-HCl (pH 8.8), 200 mM (NH4) So4, 0.1% Tween 20], 2 mM MgCl2, 250 μM of each of the four deoxynucleotide triphosphate, 1.25 U Taq DNA polymerase (Fermentas, city, Country), 50 pmol of each Species-specific oligonucleotide primers sized 171base pares (Toxo-F: 5’-CATTGGAGAGATTTGCATTC-3’) and (Toxo-R: 5’-ATCAGTATCCCAACAGAGACAC-3’) (Cinnagen, Tehran, Iran) designed from 18S ribosomal RNA gene of Toxoplasma (Accession number: JQ235842) and 5 μL of the extracted template DNA. Amplification of parasite DNA was done in (Eppendorf, Hamburg, Germany). DNA amplification was performed in a thermocycler apparatus for 33 cycles as follows: primary denaturation of the samples was performed at 94°C for 7 minutes, denaturation at 94°C for 1 minute, annealing at 56°C for 1 minute, extension at 72°C for 1 minute and final extension at 72°C for 5 minutes.
3.5. Analysis of PCR Products
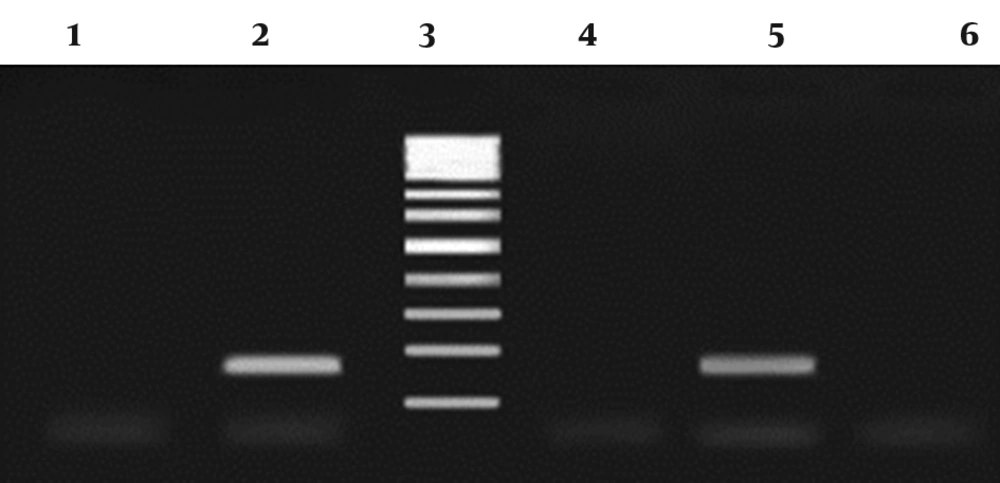
Distilled water served as the negative control. Polymerase chain reaction products were run using 1.5% agarose gel in 1X TBE buffer at 80V for 30 minutes, stained with ethidium bromide and the images were visualized in UVIdoc gel documentation systems (Uvitec, UK). The PCR products (171bp) were identified by 100 bp DNA size marker (Fermentas, Germany) (Figure 1).
3.6. Statistical Analysis
The obtained data were analyzed using SPSS (Statistical Package for the Social Sciences) software (Version 17. SPSS Inc, USA). Descriptive statistics were used to determine proportions of positive animals for T. gondii. The differences in proportions between animal species, different sexes and locations were determined by the Chi square test at P ≤ 0.05.
4. Results
In the present study a total of 155 cattle, 122 camels and 95 sheep were selected as samples from the two provinces. No evidence of contamination with T. gondii was detected in the sample cattle using PCR method. The overall prevalence of T. gondii in camels was 6.6% while the overall prevalence of the parasite in sheep was 17.9%. The distribution of T. gondii in camels and sheep in the two provinces is presented in Table 1. Table 1 also displays the infections in the different sex categories. No statistically significant differences were observed between camels and sheep, male and female camels, and male and female sheep. In sheep, however, the prevalence of T. gondii was significantly higher in Chaharmahal VA Bakhtiary (33.33%) compared to Isfahan (8.47%) (P = 0.005, 95% CI 6.88-43.35).
| Attribute | Prevalence of T. gondii | |
|---|---|---|
| Camel | Sheep | |
| Province | ||
| Chaharmahal va Bakhtiary | - | 33.33% (12/36) |
| Isfahan | 6.60% (8/122) | 8.47% (5/59) |
| Sex | ||
| Female | 5.17% (3/58) | 19.72% (14/71) |
| Male | 6.76% (5/74) | 12.5% (3/24) |
5. Discussion
The current study recorded a 0.00% prevalence of T. gondii infection in cattle (n = 155). This finding is consistent with previous reports in the country in which no evidence of T. gondii infection was noted in cattle (12, 13). Several other studies have recorded lower infection levels of T. gondii in these animal species (14-22). The obtained results, showing no or lower T. gondii infections in cattle, are in support of the hypothesis that cattle are not a favoured host for T. gondii, and that human infection is most likely associated with consumption of pig, lamb and goat meat (13, 20, 23-25). However, other studies (26-28) have obtained high levels of T. gondii infection among cattle thereby contradicting the hypothesis. Variations in the levels of infection with T. gondii among cattle in the different studies could probably reflect differences in exposure rates to the parasite, which is attributable to the contamination rate of the environment.
According to Dubey and Thulliez (29), cattle have a high natural resistance to T. gondii and infection in them does not usually cause clinical symptoms. T. gondii infections in sheep and goats are known to induce abortions, pre-term deliveries, weak newborns and neonatal mortality (30-33). This frustrates scientific endeavours of geneticists, nutritionists and livestock breeders working for the propagation of small stock. It is a drain of breeding animals and thus heightens the gap of animal proteins between an increasing human population. In the current study the prevalence of T. gondii infection in the sheep samples was at 17.90%. T. gondii infections in sheep have been also observed in a number of investigations worldwide (12, 14-16, 18, 20-22, 26-28, 34-37). Considering reproductive losses caused by the parasite, these results imply substantial economic losses to the sheep raising industry worldwide (38). Among the sample camels (n = 122) 6.60% were infected with T. gondii. A more or less similar prevalence of the parasite in camels (4.2%) was obtained in a previous investigation conducted in the country (39). High levels of infection with T. gondii among camels have been found in Saudi Arabia (40), Sudan (41) and Egypt (42). It is noteworthy that in the current study no association was found between the prevalence of T. gondii and sex, both in camels and sheep. A similar observation was made in a study conducted in Nigeria (43). However, studies in Ghana (44), Pakistan (45), Brazil (46) and China (47) revealed higher prevalence of T. gondii in female than in male sheep. Some authors have indicated that female animals are more susceptible to infections with protozoan parasites than males (48). According to Kittas et al. (49) innate immune responses are enhanced in males. A significantly higher prevalence of T. gondii was recorded in the sheep raised in Chaharmahal va Bakhtiary than the ones raised in Isfahan. This is in accordance with previous findings which also identified geographical differences in T. gondii infections among animals (18, 50-52). This observation could be an attribute of differences in levels of environmental contamination. Investigations elsewhere have attributed human population density to geographical differences in the prevalence of protozoan parasites among animals (53).
Overall, the results of the present study confirm that T. gondii is prevalent; and that the infection is widely distributed in camels and sheep in the two provinces. There is a need to investigate the potential risk factors for infection of humans with the zoonotic parasites. Zoonotic implication of the parasite associated animal health and production losses in the area under study still need to be explored.