1. Background
Uropathogenic Escherichia coli (UPEC) are one of the most important etiologic agents of urinary tract infection (UTI) (1, 2). UTI is the most frequent human bacterial infection all around the world (2). It is estimated that annually global economy spends more than six billion dollars on UTI (3). Studies show that about 50% of women and 12% of men get UTI during their life (4). Also 20% to 30% of women experience recurrent infections within 6-12 months (3, 4). Therefore, it is a major public health issue (5). Several virulence factors such as fimbriae, toxins, and siderophores contribute to the colonization and pathogenicity of UPEC (6). Virulence factors are specific traits enabling E. coli to overcome host immune system and cause various diseases (7). Virulence genes are located on transmissible genetic elements and/or in particular regions on the chromosome that are called pathogenicity islands (8). Pathogenicity islands are associated with the genome of pathogenic strains (9) and led to coordinate horizontal transfer of virulence genes between strains of one species or even related species (10).
UPEC strains also have other various types of virulence factors such as adhesins, toxins and iron uptake systems that facilitate colonization and persistence of the bacteria in the urinary tract (11). Attachment of the bacterium to the uroepethelium is the main step to initiate and develop UTI (12). Adhesins, such as p-fimbriae, help the bacteria to resist against urinary lavage and invade epithelial cells (13). P-fimbriae are one of the most important adhesions encoded by pap (pyelonephritis-associated pili) genes and could act as predictors of pyelonephritis (12). Other important virulence characteristic of UPEC is serum resistance; the ability that protects bacteria against bactericidal activity of serum (12). The gene traT is a surface exclusion protein that mediates resistance to serum (14). Identification of virulence factors can be useful for diagnosis and therapeutic strategies (15). Studies show that antibiotic resistance is increasing among UPEC strains every year (16). The high antimicrobial resistance of UPEC significantly reduces the therapeutic options and increases the treatment costs and mortality rates (17, 18). To the authors best knowledge; few data are available regarding the virulence factors and antimicrobial resistance patterns of UPEC strains in Iran.
2. Objectives
The current study aimed to investigate the virulence associated determinants as well as their patterns of antibiotic resistance in UPEC isolated from hospitalized patients with UTI.
3. Materials and Methods
3.1. Bacterial Isolates
One-hundred and fifty non-duplicate E. coli strains were isolated from urine samples of hospitalized patients with UTI at Shahid Beheshti Hospital in Kashan, Iran, from December 2012 to June 2013. A positive urine culture (≥ 105 cfu/mL) indicated UTI. The isolates were referred to the Department of Microbiology, Kashan University of Medical Sciences and identified as UPEC, using standard biochemical tests (19). All the strains were stored at -70°C in Tryptic Soy Broth (TSB) medium supplemented with 10% glycerol.
3.2. Antimicrobial Susceptibility Testing
The antibiotic susceptibility patterns were determined using the disk diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (20). The following antimicrobials were tested: amoxicillin-clavulanic acid (AMC: 20/10 µg), ampicillin (AMP: 10 μg), aztreonam (ATM: 30 μg), cefoxitin (FOX: 30 μg), ceftriaxone (CRO: 30 μg), ciprofloxacin (CIP: 5 μg), gentamicin (GEN: 10 μg), nalidixic acid (NA: 30µg), nitrofurantoin (NIT, 300 µg), ceftazidim (CAZ: 30 μg), trimethoprim-sulfamethoxazole (SXT: 25 μg) and imipenem (IMP: 10 μg). The quality control organism was E. coli ATCC 25922. Results were interpreted as susceptible or resistant according to criteria recommended by the CLSI and the manufacture protocols (Mast Companies, UK).
3.3. DNA Extraction and Virulence Genes Amplification
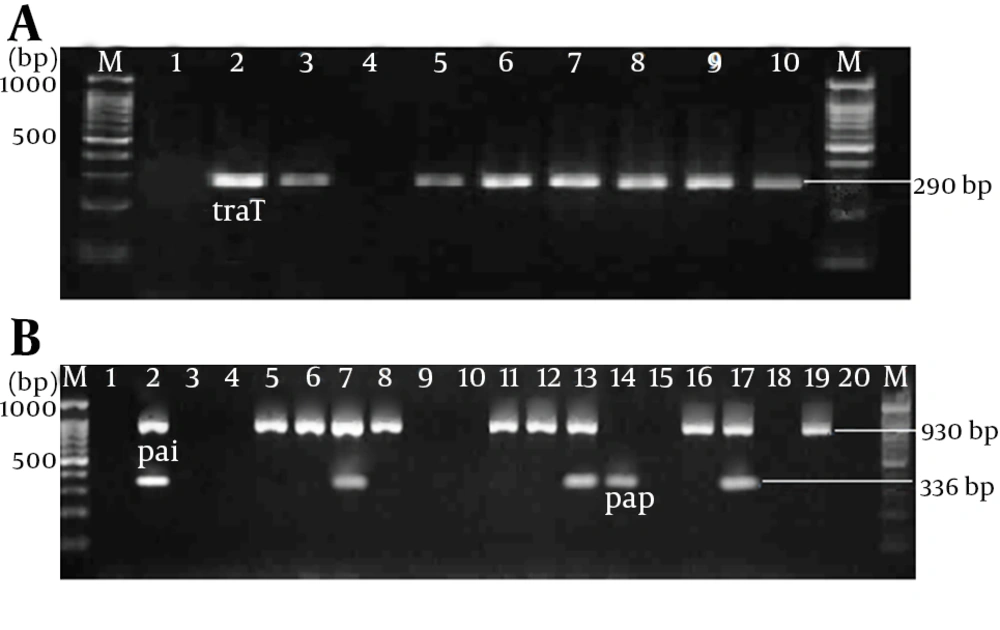
DNAs of 150 E. coli isolates were extracted using boiling method. Before DNA extraction the E. coli strains were cultured in LB broth at 37°C for 18 hours. Bacteria were pelleted from 1.5 mL LB broth and suspended in 200 µL of sterile deionized water and incubated at 100°C for 10 minutes. The supernatants were used as a template DNA after centrifugation of the lysate. PCR assays were used to detect pap (pilus associated with pyelonephritis), traT (serum resistance associated) genes and PAI marker of the pathogenicity island of the UPEC. The following primers were used to amplify virulence genes: 5′-gcaacagcaacgctggttgcatcat-3′ and 5′-agagagagccactcttatacggaca-3′ for pap gene to detect a 336-bp amplicon, 5′- ggtgtggtgcgatgagcacag-3′ and 5′- cacggttcagccatccctgag-3′ for traT gene to detect a 290-base pair amplicon, and 5′-ggacatcctgttacagcgcgca-3′ and 5′ - tcgccaccaatcacagccgaac-3′ for PAI marker to detect a 930-bp amplicon (10). The virulence genes were amplified in a total volume of 25 µL including 5 µL of template DNA, 2.5 μL of 10 × reaction buffer, 1 µL (10 pmol) of each of the forward and reverse primers, 0.5 µL of 200 µM of dNTP, 1.5 mM MgCl2, and 1.25 U Taq DNA polymerase. PCR condition was as follows: 2 minutes at 94ºC and 30 cycles including denaturation at 94°C for 60 seconds, annealing at 63°C for 30 seconds, extension at 72ºC for 90 seconds and 72ºC for 5 minutes for the final extension in a Thermal Cycler apparatus (Eppendorf master cycler, MA, Germany). The PCR products were electrophoresed on 1.8% agarose gels. The gels were stained in ethidium bromide for 15 minutes and visualized in gel document system (Biorad, UK). The sizes of the DNA bands were determined by comparing them with a 100-bp DNA ladder as the molecular size marker (100 bp DNA ladder, MBI Fermentas).
3.4. DNA Sequencing
The purified PCR products were sequenced using the ABI Capillary System (Macrogen Research, Seoul, Korea). Sequences were compared using online BLAST software (http: //www.ncbi.nlm.nih.gov/BLAST/).
4. Results
A total of 150 E. coli strains were isolated from patients admitted to the various wards of Shahid Beheshti Hospital, Kashan, Iran, were analyzed. Urine samples were from patients of both genders (78% females, 22% males) aged between 1-95 years, and the mean age of 50 years. The morphology exhibited gram-negative short rod bacteria arranged in single or paired forms. In biochemical examination all the isolates revealed positive reaction in Indole and Methyl Red (MR) tests, negative reaction in Voges Proskauer (VP) and citrate utilization tests.
4.1. Antimicrobial Susceptibility Profile
The results of antibiotic susceptibility testing are shown in Table 1. Of the 150 E. coli strains isolated from UTI, 9 (6%) were susceptible to all tested antimicrobials tested whereas 111 (74%) showed resistance to more than three antimicrobial families and identified as multidrug-resistant (MDR). Fifty-eight (38.7%) isolates were resistant to three to six antibiotics, and 53 isolates (35.3%) were resistant to more than seven antibiotics. One isolate was resistant to imipenem and 84 (56%) isolates demonstrated resistance to the extended spectrum β-lactam antibiotics in disk diffusion test. The highest resistance was observed against ampicillin and cotrimoxazole.
4.2. Prevalence of Virulence Genes Among UPEC Isolates
Totally, the virulence genes were detected in 126 (84%) UPEC isolates; however in 24 strains (16%) none of these virulence genes were found. Thirty-nine strains (26%) had only one virulence gene, 72 strains (48%) two genes; combinations of three virulence genes were observed in 15 (10%) strains. The frequencies of the studied virulence genes are reported in Table 2. Also PCR products of pap and traT genes and PAIs markers after electrophoresis on agarose gels are shown in Figure 1. According to the virulence determinants, the traT gene was the most common virulence gene and was detected in 111 (74%) of the isolates. The PAIs markers and the pap gene were found in 92 (61.3%), and 25 (16.6%) of the isolates, respectively (Table 3). The virulence associated genes were distributed in eight distinct patterns that the most common pattern was traT positive, PAI positive, and pap negative (Table 4). BLAST software was employed to analyze and sequence the results of purified PCR products. It revealed that PCR products of 336 bp, 290 bp and 939 bp amplicons were papE allele, traT gene and PAI ICFT073 marker, respectively.
| Antibiotic | Isolates | ||
|---|---|---|---|
| Sensitive | Intermediate | Resistant | |
| Ampicillin | 23 (15.3) | 5 (3.4) | 122 (81.3) |
| Nalidixic acid | 42 (28) | 1 (0.7) | 107 (71.3) |
| Cotrimoxazole | 49 (32.7) | 4 (2.7) | 97 (64.7) |
| Ciprofloxacin | 55 (36.7) | 3 (2.0) | 92 (61.3) |
| Ceftriaxone | 65 (43.3) | 0 (0) | 85 (56.7) |
| Aztreonam | 71 (47.3) | 2 (1.3) | 77 (51.4) |
| Ceftazidim | 73 (48.7) | 3 (2.0) | 74 (49.3) |
| Gentamicin | 80 (53.3) | 10 (6.7) | 60 (40.0) |
| Nitrofurantoin | 115 (76.6) | 9 (6.0) | 26 (17.3) |
| Amoxicillin-clavulanic acid | 107 (71.3) | 9 (6.0) | 24 (16.0) |
| Cefoxitin | 120 (80.0) | 2 (1.3) | 28 (18.6) |
| Imipenem | 145 (96.7) | 4 (2.6) | 1 (0.7) |
aData are presented as No (%).
Electrophoresis of PCR Product on 1.8% Agarose Gel; (a) Lanes M: 100-bp DNA ladder as the molecular size marker; lane 1: PCR mix with no template (negative control); lane 2: positive control for traT gene; lanes 3 and 5- 10: the traT gene was detected in UPEC strains; lane 4, traT -negative UPEC strain. (b) Lanes M: 100-bp DNA ladder as the molecular size marker; lane 1: PCR mix with no template (negative control); lane 2: positive control for pap and pai genes; lane 3-4, 9-10, 15, 18 and 20: the pap and pai negative UPEC strains; lanes 5-6, 8, 11-12, 16, and 19: pai-positive UPEC strains; lane 14: pap-positive UPEC strain; lanes 7, 13 and 17: UPEC strains that were positive for both pap and pai genes.
| Virulence Gene | Positive isolates | Negative Isolates | Total |
|---|---|---|---|
| pai | 92 (61.3) | 58 (38.7) | |
| pap | 25 (16.6) | 125 (83.4) | |
| traT | 111 (74) | 39 (26.0) | 150 (100) |
aData are presented as No (%).
| Virulence Pattern | Virulence Gene | Strains | ||
|---|---|---|---|---|
| Pai | pap | traT | ||
| UPEC1 | - | - | - | 24 (16.0) |
| UPEC2 | - | - | + | 30 (20.0) |
| UPEC3 | + | - | - | 9 (6.0) |
| UPEC4 | - | + | + | 1 (0.7) |
| UPEC5 | + | - | + | 62 (41.3) |
| UPEC6 | - | + | + | 4 (3.4) |
| UPEC7 | + | + | - | 6 (2.7) |
| UPEC8 | + | + | - | 14 (9.3) |
5. Discussion
Escherichia coli is the cause of more than 80% of urinary tract infections in all age groups and can lead to renal failure if remained untreated for a long time (2, 21). Various virulence factors can be attributed to UPEC pathogenicity (5). Better knowledge of the properties of virulence and its antibiotic resistance pattern helps clinicians to anticipate the development of infection in the patients. Surface virulence factors of UPEC including adhesions, are responsible for colonization of bacteria in the urinary tract. The current study results showed that 16.6 % of UPEC isolates carried pap gene. The frequency of pap gene in UPEC isolates in the present study was lower compared to those of found in Brazil, Tunisia, China, (5, 6, 22), but is in agreement with the results of studies conducted by Usein et al. and Santo et al. who reported the gene frequencies of 17% and 14%, respectively (14, 21).
According to the literature, the frequency of pap gene can vary from 0% to 77% (5, 8, 10, 11). The diversity in frequency of pap gene among different studies can be due to the fact that UPEC strains utilize a variety of adhesins to bind to the urinary epithelial cells, and start the infection. Hence, the strains lacking the pap operon may use other adhesins encoding operons such as afa, and sfa for binding. The PAI marker showed a frequency of 61.3% in the current study. Johnson et al. (10), reported a frequency of 71% for PAI - markers among UPEC isolated from the patients with urosepsis in the USA. In a survey conducted by Navidinia et al. (23), a prevalence of 89% was documented for these markers in UPEC strains. It is documented that the pathogenicity islands (PAIs) capable of horizontal virulence genes transfer between species; therefore, a frequency of 61.3% for PAI markers in UPEC strains isolated from hospitalized patients is notable.
Usually, the invasive pathogens are highly resistant to the lethal activity of serum and the role of traT- protein in resistance of bacteria to serum is very important. The current study results showed that 74% of UPEC isolates contained traT gene. A study in the USA on blood samples from patients with urosepsis showed that traT is a common gene among immune-compromised patients (10). Oliveira et al. (11) showed that 76%of the urine samples were contaminated with multidrug-resistant bacteria carrying traT gene and Kudinha et al. reported a frequency of 77% (2) for traT gene among E. coli strains isolated from patients with cystitis. These results are compatible with those of the current study, which suggest that the traT, as a common and important virulence factor, could be considered as a target for therapeutic interventions. In the current study, eight distinct patterns were identified among the UPEC strains; whereas, high heterogeneity of the virulence genes compositions were documented in other studies (10, 11).
Urinary tract infection is one of the most common bacterial infections and increased antibiotic resistance has complicated the treatment of such infections. Seventy-four percent of UPEC strains demonstrated multidrug-resistance phenotype and showed resistance to more than three of the tested antimicrobials in the present study. High frequency of antibiotic resistance among UPEC strains were reported in previous studies in Iran (8, 24). Johnson et al. (10) reported 50% multidrug-resistant E. coli among the species isolated from urine specimens. Finding of the current study showed a high rate of resistance to ampicillin, cotrimoxazole, nalidixic acid and ciprofloxacin. Studies from other countries also reported the high frequency of resistant to antibiotics in E. coli strains isolated from urine samples (8, 25-27).
Treatment failure with the first line drugs like ampicillin, cotrimoxazole, nalidixic acid has led to excessive prescription of floroquinolons and extended spectrum cephalosporins for treatment of wide range of infections including UTI, gastroenteritis, lower respiratory tract infection, and other infections in Iran. Floroquinolons, including ciprofloxacin, are not recommended as first-line antibiotics for the treatment of UTI, but they are generally advised for the empirical therapies. Regarding the results of the current study, 61.3% resistance to ciprofloxacin, which may result from discriminate use of this antibiotic in Iran, is considerable. Also, resistance to third generation cephalosporins, such as ceftazidim, and ceftriaxone was high in the present study. These results corresponded to other studies conducted by Rezaee et al. (24) and Mukherjee et al. (27) who reported 46.4% and 55% resistance in E. coli to ceftazidime, respectively; but are in contrast with Oliveira et al. (11) findings who documented 1% ceftriaxone-resistant UPEC.
Resistance to extended spectrum cephalosporins has increasing considerably in developing countries like Iran, and it was primarily due to the ample and discriminate use of antibiotics in these countries. In the present study, 16% of the UPEC strains showed resistance to amoxicillin-clavulanic acid. Similar results obtained from other studies (28, 29). In Mexico, the rate of resistance to amoxicillin-clavulanic acid in E. coli strains isolated from outpatients with uncomplicated UTI was 19.6% (28). In Another study on community-acquired urinary tract infections in Italy, the rate of amoxicillin-clavulanate-resistant E. coli isolates was 18.2% (29). Because of the relatively low antibiotic resistance rate, this drug can be recommended for the treatment of urinary tract infections. According to the findings of the current study, UPEC strains showed high sensitivity to nitrofurantoin, which confirms the results of other studies (30, 31). Sensitivity to nitrofurantoin may result from decreasing the use of this drug (32). As nitrofurantoin is unable to create appropriate level in blood flow, it is used only for treatment of uncomplicated UTIs, and not recommended for complicated UTI and systemic involvements.
The present study showed high resistance to aztreonam, which was in accordance with the findings of Navidinia et al. who demonstrated that 78% of UPEC strains were resistant to aztreonam (23). High resistance to aztreonam was reported by Pobiega et al. among the extended-spectrum β-lactamases (ESBL)-producing E. coli strains (33). Aztreonam is not used in Iran. Resistance against aztreonam may be associated with the production of ESBL enzymes by ESBL-producing strains, which breakdown beta-lactam ring in this antibiotic. Identifying one imipenem resistant UPEC and four isolates with reduced susceptibility to this antibiotic were other interesting findings of the current study. Most studies have reported 100% sensitivity to imipenem (1, 31), but the resistance to imipenem in E. coli strains isolated from urine samples was indicated in other studies (34). Rezaee et al. reported 1.4% resistance to imipenem among E. coli isolates (24). As carbapenems are advising for the treatment of severe infections caused by ESBL-producing E. coli, emerging resistance to this antibiotic is a place of major concern in treating infections caused by such resistant bacteria.
In conclusion, finding of the current study showed that the traT gene and PAI markers were highly prevalent among UPEC strains isolated from hospitalized patients in Kashan; hence, they could be considered as useful targets for prophylactic interventions. Also, in the current study high resistance was observed against the antibiotics widely used for the treatment of urinary tract infection; therefore, to reach better therapeutic outcomes, empiric treatment regimens have to be modified.