1. Background
Although many etiological agents such as trauma, vaccination, radiotherapy, hormonal factors and infections have been proposed for morphea (localized scleroderma), the main causative agent is still unknown. In 1985, spirochaetal etiology was suggested for morphea (1). This hypothesis drew great attention as it necessitated antibacterial treatment for morphea. In the following years, serological, immunohistochemical and culture approaches were performed to determine the role of Borrelia burgdorferi in the pathogenesis of morphea (2-5). However, these studies concerning the relation between B. burgdorferi and morphea have had conflicting results. In recent years, several authors have used polymerase chain reaction (PCR) to detect B. burgdorferi in skin lesions of patients with morphea, but the relationship between morphea and this organism has still remained controversial (6-10). The most acceptable reason for this confliction is the possible geographical variations and different Borrelia subspecies which affect the relationship, as Borrelia has frequently been detected in European and Asian patients, but not in cases from the USA or Scotland (10-13).
2. Objectives
The present study was designed to record the occurrence of B. burgdorferi among skin biopsies of patients with morphea. It was the first study in our country. If the link is firmed, the next studies can focus on the role of antibiotic therapy in morphea.
3. Patients and Methods
This was a case series study performed on skin biopsy specimens of patients with clinical and pathological diagnoses of morphea. By reviewing the records of the Pathology Department of Imam Reza and Ghaem hospitals as the two main university hospitals in northeast of Iran during October 2003 to October 2009, all the patients with histological compatibility with morphea were selected. Sixty six patients with morphea were prospectively included in the present study. After reconfirmation of histological diagnosis, five to eight sections (5 µm) were cut from each formalin-fixed, paraffin-embedded (FFPE) tissue block and were deparaffinized by adding xylene (Sigma-Aldrich, LLC). Thereafter, DNA was extracted from those tissue samples using commercial DNA isolation kit for FFPE (QIAamp DNA FFPE Tissue Kit; Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions (QIAamp DNA FFPE Tissue Procedure).
The quality of the DNA sample and the absence of PCR inhibitors were checked in all the samples by amplification of a part of the human b-actin gene. Afterwards, the presence of Borrelia DNA was tested by PCR in a Techne gradient thermal cycler (TC-5000 gradient thermal cycler, Techne, UK). This study was performed by a PCR kit (GenePak DNA PCR test, Isogene Lab Ltd. Russia) which could detect three species of Borrelia (B. burgdorferi, B. garini, B. afzelii) (14).
Five microliters of each DNA sample was added using a hot-start technique. The amplification condition comprised an initial denaturation step of two minutes at 95°C and then 43 cycles as one minute at 95°C, 50 seconds at 58°C, and one minute at 72°C, with the final extension step prolonged to two minutes at 72°C to ensure complete amplification of the target. Positive and negative controls were included in each batch of amplifications. Electrophoresis in 1% agarose gel (Sigma-Aldrich Co. LLC. Germany) was used for the analysis of the PCR products. PCR products were visualized by ethidium bromide (ETBr) staining under UV light. Visualizing the 445-bp fragment was interpreted as positive result. Precautions to avoid cross-contamination were taken in every assay (15).
4. Results
The patients consisted of 40 females (60.6%) and 26 males (39.4%), with a mean age of 28.39 ± 17.28 years. The youngest patient in this study was one and the oldest was 76 years old at the diagnosis time. The mean duration from the onset of lesions to the time of biopsy was 15.5 ± 23.86 months. In 26 patients (39.4%), the lesions had appeared during the last six months, indicating an active process. Biopsies were taken mainly from the trunk (n = 38, 57.6%) and lower extremities (n = 14, 21.2%); 7 (10.6%) were from the upper extremities and 7 (10.6%) from the head and neck area. The lesions were more frequent on the trunk (abdomen, n = 6; back, n = 18; chest, n = 10; flank, n = 4), than leg (n = 14), arm (n = 7), head (forehead, n = 4; scalp, n = 2) and neck (n = 1). The results are shown in Table 1.
| Lesion Localization | Female | Male |
|---|---|---|
| Trunk | 25 (62.5) | 13 (50) |
| Lower extremities | 6 (15) | 8 (30.8) |
| Upper extremities | 4 (10) | 3 (11.5) |
| Head and neck | 5 (12.5) | 2 (7.7) |
a Data are presented as No. (%).
According to the classification of Peterson, the clinical manifestations of morphea were of the plaque type in 59 (89.4%), linear in 6 (7.6%) and frontoparietal morphea in 2 (3%) patients (16). Results are shown in Table 2.
| Clinical Manifestations | Female | Male |
|---|---|---|
| Plaque morphea | 39 (97.5) | 20 (76.9) |
| Linear morphea | 0 (0) | 5 (19.2) |
| Frontoparietal morphea | 1 (2.5) | 1 (3.8) |
a Data are presented as No. (%).
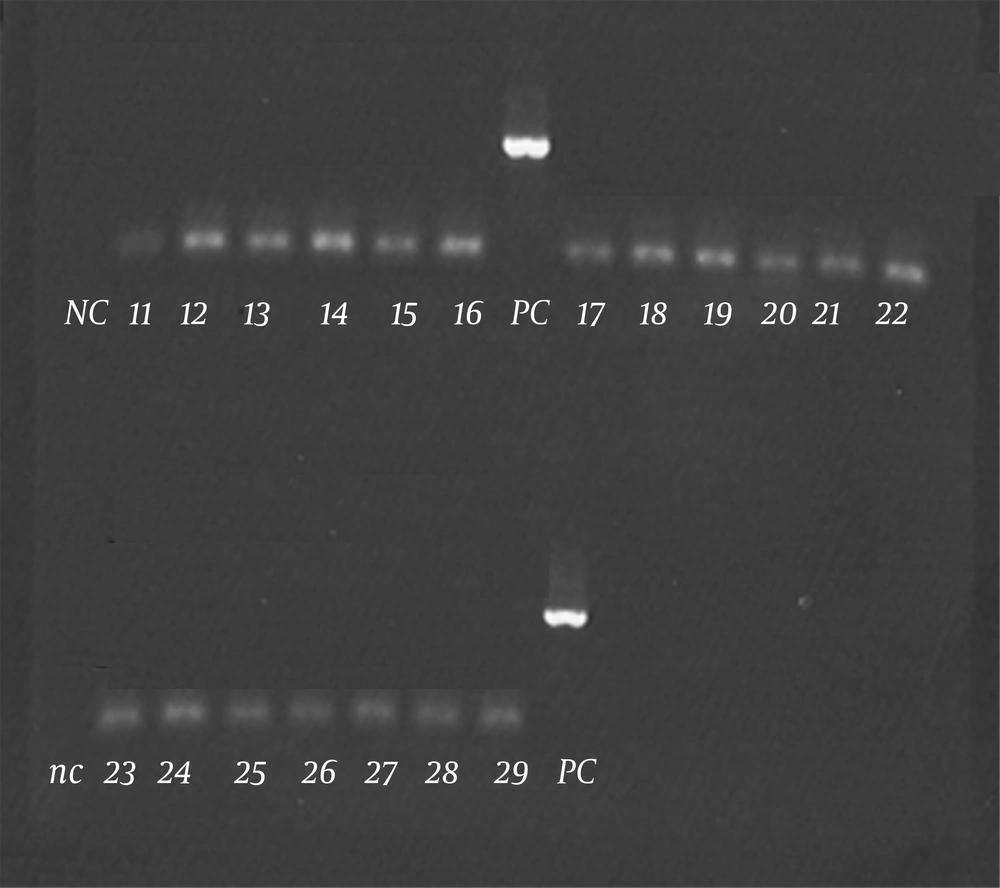
Isolation of sufficient DNA with regard to quality and quantity was shown in all the clinical specimens by successful amplification of a part of the human b-actin gene. No Borrelia DNA was detected in skin biopsies of 66 patients with morphea with PCR (Figure 1).
5. Discussion
The present study was designed to determine the frequency of Borrelia spp. in morphea lesion in our region and showed no relationship between Borrelia infection and development of morphea. PCR was performed in 66 cases with confirmed morphea to detect Borrelia DNA in skin biopsies. All the 66 cases were negative for Borrelia-specific DNA, despite successful amplification of appropriate positive controls in every test, demonstrating the lack of evidence for an association between Borrelia infection and morphea. In reviewing other studies, initial studies used serological methods for investigation the correlation between Borrelia infection and morphea. In several studies, all the tested patients with morphea were seronegative, while some other studies found specific antibodies against Borrelia in 6 - 54% of unselected patients with morphea (2, 5-8, 17-26). Since negative serology does not exclude previous infection with Borrelia and positive serology may merely represent coincidental infection, other studies sought more definite evidence of a causal link by seeking to demonstrate the organism in biopsies of skin lesions taken from patients with morphea.
Attempts to visualize Borrelia–infected organisms directly in histological sections after appropriate staining have demonstrated spirochaetes in only a small number of cases (2, 3, 19-21, 23, 27, 28). Several studies using culture of Borrelia from biopsies of morpheic lesions have shown completely negative results (2, 17, 18, 25, 27), while in a small number of cases positive results have been achieved (4, 20, 22, 23, 28). In view of these conflicting results, recent studies have focused on PCR techniques to demonstrate the organism. Once more the results have been contradictory. Studies reporting a positive association between Borrelia infection and morphea have shown evidence of the organism in 26 - 100% of cases (8, 10, 26, 29, 30), whereas in further 10 reports including our current one, no positive case has been identified (5, 7, 9, 11-13, 18, 25). Isolated studies have reported a positive association in countries such as Italy, Switzerland, Puerto Rico, Turkey, and Japan, and negative association in Spain, Finland, Holland, the USA, some parts of Germany and France (2, 3, 10, 18, 25-27, 29, 30).
All the PCR examinations were thoroughly controlled by the use of positive and negative controls. Based on these procedures, there is a high probability that we would have been able to detect Borrelia DNA, had it been present. Fujiwara and coworkers suggested that morphea might be caused by certain subspecies of B. burgdorferi which are endemic, exclusively in certain geographical areas (10). Since the literature suggests that there is a strong geographical relation between Borrelia infection and morphea, the results of the present study, which was the first study concerning the association between infection with Borrelia spp. and morphea in northeast of Iran, suggests that morphea is probably not associated with Borrelia spp. in northeast of Iran. These results indicate that in northeast of Iran, there is no association between infection with Borrelia spp. and the subsequent development of morphea. This could explain why all patients do not benefit from antibiotic therapy. The reason for the inconsistent results in studies using PCR could be the low number of microorganisms found in the tissue, ie, below the detection threshold for this technique (5-7, 11, 25, 31). Other explanations include previous antibiotic treatment, old stage of the disease, wrong biopsy site (eg, from the negative sclerotic area), or wrong fixation of tissue specimens leading to DNA cross-linking (e.g., with inadequately buffered formalin). In this study, we used FFPE tissue blocks, so the abovementioned points could have caused some limitations in our study.
In conclusion, PCR examination showed no evidence for Borrelia infection in our group of patients with morphea. Therefore, we found consistent evidence for absence of B. burgdorferi infection in patients with morphea in northeast of Iran. The result of this study showed no relationship between Borrelia infection and morphea lesions and in other word indicated that morphea, at least in Iran, is not caused by Borrelia spp. This study proposed a geographical relationship between different Borrelia subspecies as causative agents of morphea, which was absent in our region. If the link is firmed, the next studies can focus on the role of antibiotic therapy in morphea.