1. Background
Hepatitis G Virus (HGV) belongs to the Flaviviridae, which includes three genus and more than 70 members. Hepatitis G Virus members are widely variable and biologically different (1, 2). Despite the gene structure and duplication similarities, there is no antibody cross-reactivity among HGV members proteins (3, 4). Hepatitis G Virus is an envelope and spherical shaped virus of 40 - 60 nm diameters that E-protein, the most important protein of HGV, is necessary for the virus adhesion and fusion (5); therefore, determination is important in case of the anti-E2 antibodies presence. The HGV genome is composed of a single stranded RNA with the length of 11 kb, caped on 5' without poly-A tail at the 3' end (6). The HGVs isolated from different geological locations are genetically variable (7). Hepatitis G Virus cannot be cultivated and a sensitive and suitable cell type of its culture is not developed yet.
Diagnosis of HGV is according to the Revers Transcription Polymerase Chain Reaction (RT-PCR) and Enzyme Linked Immunosorbent Assay (ELISA) in biological samples; however, RT-PCR technique is valuable to detect current infections (8). Two different techniques, RT-PCR and ELISA, consider different targets to diagnose HGV; RT-PCR only detects HGV RNA molecules in the patient samples, but ELISA measures antibodies against E2-proteins. Therefore, a patient may have antibody titers for E2 proteins but its RT-PCR result may became negative because of an active immune response. Prevalence of HGV varies in blood donors ranging from 0.9% to 10%. Besides blood products transfusion, other routes for transfection include placental and needle sticking, especially for drug users (8-16). Hepatitis G Virus is mostly concomitant with hepatitis B and C viruses (HBV and HCV). Anyway, HGV has no definitive impact on the patient status (17, 18). However, there are reports on the pathogenesis of HGV that make the prevalence studies essential, especially for healthcare providers and authors. According to the reports by the investigators, HGV could develop fulminant hepatitis, which its causes are manifested sporadically (19-28). Since the patients with renal failure who undergo dialysis receive blood products and transfusion, the current study measured the prevalence of HGV assay in the patients undergoing hemodialysis and kidney transplantation in Khuzestan province, Iran.
2. Objectives
The current study aimed to investigate the prevalence of HGV using determination of E2, viral envelope antigen, antibodies and its RNA by ELISA and RT-PCR techniques.
3. Patients and Methods
To evaluate the prevalence of HGV antibody and RNA, 516 serum samples were collected from the patients undergoing hemodialysis and kidney transplanted and stored at -70 °C until the test running day. Also other data including gender, hospitalization or outdoor monitoring, and place of residence were recorded for each patient; 86 cases were from Ahvaz Kidney Transplantation Center and the rest were from other cities of Khuzestan province; also 60 sera belonged to the patients undergoing hemodialysis from Ahvaz. All patients were oriented and informed with the study purpose and signed written consent letter. Furthermore, this study was reviewed, accepted and approved by the Ethical Committee of Ahvaz Jundishapur University of Medical Sciences.
3.1. Enzyme Linked Immunosorbent Assay
To diagnose seropositive patients, ELISA tests were performed using Diagnostic Kit (Diaplus Inc., USA). The test evaluates immunoglobulin G (IgG) developed against HGV protein E2. The test was performed according to the manufacturer’s instruction and the steps briefly were: serums were diluted by sample buffer (1/10 ratio), the diluted samples were added to wells and stored at 37 °C for 30 minutes and then washed with antihuman antibody and conjugated with horse radish peroxidase (HRP) and again storing at 37 °C. After well rewashing, substrate was added and the chromogenic reaction was blocked by stopping solution. Optical density of each sample was measured in 450 nm and 630 nm as reference filter. To evaluate HBV and HCV involvement in the patients, serological determination was done using hepatitis B surface antigen (HBs-Ag) and HCV-Ab ELISA kits (Diaplus, USA) according to the kit manufacturers.
3.2. Reverse Transcription-Polymerase Chain Reaction
To measure HGV RNA positive/negative in patients, sera RT-PCR was performed. To extract viral RNA, Tripure RNA Extraction Kit (Roche, Germany) and to synthesize cDNA Sensiscript kit (Qiagen, USA) were used. Briefly, after viral RNA extraction, microtubes were kept at 65 °C for five minutes and transferred into the ice cold dishes to prepare cDNA as template RNA. Then cDNA synthesis was performed as follows: RT buffer 2 µL, dNTPs (5 mM) 2 µL, Random Primer (10 picoM) 2.5 µL, RNAsin (10 U/µL) 1 µL, Reverse Transcriptase 5 µL, Distilled Water 6.5 µL were added, then mixed and incubated at 37 °C for one hour.
cDNA synthesis was derived according to the manufacturers instruction in a reveres transcription reaction. Then the final product of cDNA synthesis was amplified by nested PCR. Primers used for the first step of PCR were 58 (58F-5' CAG GGT TGG TAG GTC GTA AAT CC-3') and 75 (75R-5' CCT ATT GGT CAA GAG AGA CAT-3'). The first step was performed as follows: PCR buffer 5 µL, dNTPs (5 mM) 1 µL, forward primer (58F) 1 µL, reverse primer (75R) 1 µL, Taq-polymerase 0.3 µL, cDNA 5 µL, and distilled water 36.7 µL were added; then Thermocycler apparatus (Techne, UK) was programmed as follows: 94 °C for 30 seconds, 60 °C for 30 seconds, 72 °C for 30 seconds in 30 cycles. In the second step, 5 µL of the first step final product was used with 131 (131F-5'AAG AGA GAC ATT GAA GGG CGA CGT-3') and 134 primers (134R- 5' GGT CAT CTT GGT AGC CAC TAT AGG-3'). The reaction was performed for 25 cycles. Ultimately, 5 µL of amplified product was added to the wells of a 2% gel agarose and the electrophoresis was derived at 100 V for one hour. The PCR final product immersed into a dish containing 20 µL ethidium bromide in 200 mL distilled water. Transiluminator apparatus (Vilber-lurmat, French) was used to visualize the bands and imaging. The 208 base pair amplicon was estimated in comparison with the DNA ladder.
3.3. Statistical Analysis
Statistical analysis was conducted using Chi-square test, and the prevalence determination of each investigated variable; the confidence interval of 95% (CI = 0.95) was considered to estimate significant results. Data were recorded and analyzed using SPSS software version 16.
4. Results
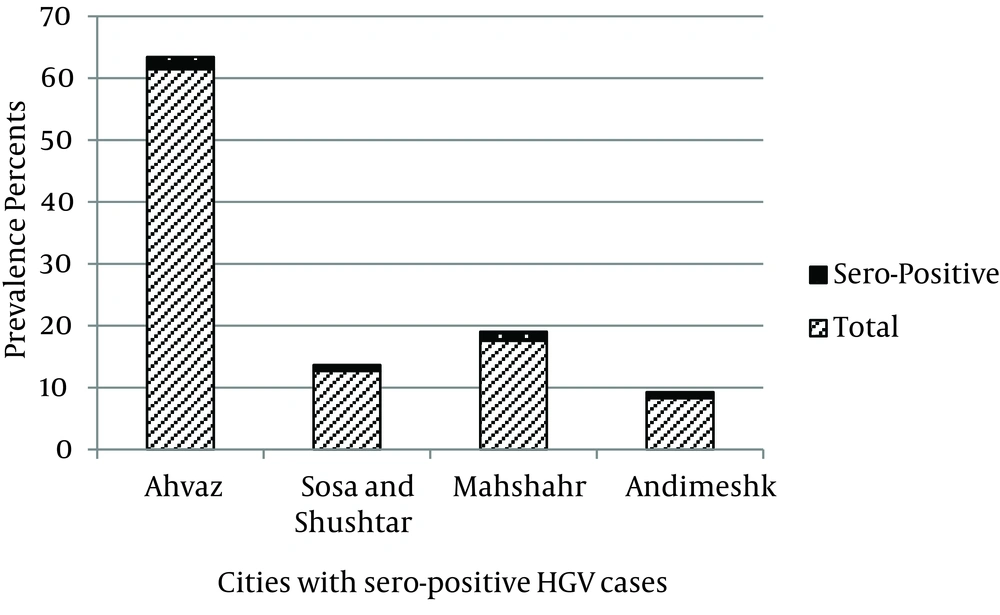
ELISA and RT-PCR tests were performed on 516 sera gathered from different cities of Khuzestan province to evaluate the prevalence of HGV. Table 1 shows the obtained results of the two evaluating techniques. According to ELISA test results for different cities, from 126 samples of Ahvaz hospitals, 4 (1.95%) samples were positive for HGV antibody. From 26 samples of Sosa and Shushtar cities, each had one positive sample. From 36 samples of Mahshahr, and 17 samples of Andimeshk, three and two samples were positive, respectively (Figure 1). All other samples from Masjid Soleiman, Shadegan, Dezfoul, Khoramshahr, and Baghmalek were negative for E2 antibody. Distribution of HGV was significantly different among the different cities (CI = 0.95, P = 0.004).
Out of the 516 sera, 285 (55.23%) samples were from males and 231 (44.77%) samples from females. Positive and negative cases of E2 antibodies between males and females are presented in Table 2. There was no significant difference between gender of the patients considering HGV antibody (CI = 0.95, P = 0.313). For the association between blood receiving frequency and HGV antibodies, data showed no considerable difference (Mean ± SD = 23.68 ± 15.9; CI = 0.95, P = 0.99); note that the minimum data is not shown.
Concurrent infection of HGV with HCV or HBV was also investigated using ELISA test for HCV antibody and HBS antigen. 438 sera were negative for both HGV and HCV and from the rest of 516 samples, 40 cases were HCV positive and HGV negative; 37 sera were HCV negative and HGV positive. Only one sample was positive in both HGV and HCV, which was considered as co-infection. From the 516 sera, 470 samples were both HBV and HGV negative; eight HBV positive and HGV negative; 36 sera were HBV negative and HGV positive; however, two samples were positive for both HBV and HGV.
Patients with transplantation were more positive for HGV immunoreactive antibodies in comparison with the renal failure ones (P = 0.000; CI = 0.95). Furthermore, Likelihood Ratio (LR) was 25.116, which implied the conclusive increase of disease likelihood in patients with transplantation. High value of LR also implied the importance of performing HGV immune-assay for kidney donors. However, calculated odd’s ratio for the patients was 0.178 (0.95 CI = 0.089 - 0.353), which implied that the chance of HGV positivity, using immunoassay, was higher among patients with transplantation but lower in patients with renal failure. In fact, patients with transplantation were 16% positive for HGV antibody; meanwhile other patients with renal failure were only 3.3% positive (Table 3).
Patients with transplantation were more positive for HCV immunoreactive antibodies compared to the ones with renal failure (P = 0.001; CI = 0.95). Furthermore, LR was 13.475, which implied the conclusive increase of disease likelihood in patients with transplantation. High value of LR also implies the importance of performing HCV immune-assay for kidney donors. Calculated odd’s ratio for negative/positive patients was 7.381 (0.95 CI = 1.76 - 30.952), which implied that the chance of HGV positivity, using immunoassay, was lower in patients with transplantation but higher in those with renal failure. In fact, patients with transplantation were only 1.3% positive for HGV antibody compared to other patients with renal failure who were 9.1% positive (Table 4).
Prevalence of HBV antibody positivity was insignificantly different between patients with transplantation and those with renal failure (P = 0.763; CI = 0.95). Likelihood Ratio for HBV ELISA test was 0.088, which implied conclusive decrease of disease likelihood in patients with renal failure. Lower values of LR also emphasized that performing HBV ELISA test was not essential for patients with renal failure; this is because of vaccination or higher rate of blood transfusion episodes in such patients. Furthermore, the obtained odd’s ratio for HBV ELISA test in patients with transplantation and those with renal failure was 0.811, and the chance of negative/positive result could range from 0.207 to 3.177 (Table 5). RT-PCR results showed a negative reaction for all 38 serum samples which were seropositive for HGV antibody; 478 samples were negative for HGV RNA, and 16 (3.14%) were positive for RT-PCR assay. Considering the low frequency of RT-PCR positive cases, the frequency was not reported for each city.
| ELISA and PCR Negative Sera | PCR Positive Sera | ELISA Positive Sera | Total Numbers |
|---|---|---|---|
| 462 (89.51) | 16 (3.14) | 38 (7.36) | 516 |
a Data are presented as No. (%).
| Serological Response | Positive | Negative | Total | P Value b |
|---|---|---|---|---|
| Gender | 0.313 | |||
| Female | 12 (5.19) | 219 (94.81) | 231 (100) | |
| Male | 26 (9.12) | 259 (90.88) | 285 (100) | |
| Total | 38 (7.36) | 478 (92.64) | 516 (100) |
a Data are Presented as No. (%).
b Chi-square; (CI = 0.95).
| Renal Failure / Transplantation | HGV ELISA Test Result a | P Value | Odd’s Ratio (for Negative/Positive) | Likelihood Ratio | |
|---|---|---|---|---|---|
| Negative | Positive | ||||
| Transplantation | 126 (84) | 24 (16) | 0.000 | 0.178 | 25.116 |
| Hemodialysis | 414 (96.7) | 14 (3.3) | (0.95 CI = 0.089 - 0.353) | ||
a Data are Presented as No. (%).
| Renal Failure / Transplantation | HCV ELISA Test Result a | P Value | Odd’s Ratio (for Negative/Positive) | Likelihood Ratio | |
|---|---|---|---|---|---|
| Negative | Positive | ||||
| Transplantation | 148 (98.7) | 2 (1.3) | 0.001 | 7.381 (0.95 CI = 1.76 - 30.952) | 13.475 |
| Hemodialysis | 391 (90.9) | 39 (9.1) | 0.001 | 13.475 | |
a Data are Presented as No. (%).
| Renal Failure / Transplantation | HBV ELISA Test Result a | P Value | Odd’s Ratio (for Negative/Positive) | Likelihood Ratio | |
|---|---|---|---|---|---|
| Negative | Positive | ||||
| Transplantation | 147 (98) | 3 (2) | 0.763 | 0.811 (0.95 CI = 0.207 - 3.177) | 0.088 |
| Dialysis | 423 (98.4) | 7 (1.6) | 0.763 | 0.088 | |
a Data are Presented as No. (%).
5. Discussion
Since 1995 that Hepatitis G Virus was identified for the first time, a series of studies considered the epidemiology and diagnosis of this virus and its pathogenesis, especially in hepatitis. Different social groups were tested for HGV and healthy blood donors, patients undergoing dialysis or kidney transplantation recipients, patients with acute, chronic, idiopathic hepatitis, and those with cirrhosis or cancer were included. Contemporary studies considered Khuzestan province patients undergoing hemodialysis therapy or kidney transplantation. All patients were subjected to two techniques; ELISA, a serological test to measure HGV antibody in the serum samples; and RT-PCR that is a molecular test to search viral RNA in the serum samples. All the sixteen sera that were positive using RT-PCR belonged to the patients undergoing hemodialysis (3.14% of all the patients undergoing hemodialysis).
The prevalence of HGV in patients undergoing kidney transplantation was reported 24% in Italy (18). The frequency of HGV is reported 50% between the patients undergoing hemodialysis in Germany (19), 12.8% in Brazil (20), 4.5% in Japan (21) and 17% in Taiwan (22). Reported data showed a 24.3% frequency of HGV among the patients undergoing hemodialysis in South Africa (15). The present study showed a 3.7% frequency of HGV among the patients undergoing hemodialysis in Khuzestan province, which was close to the Japanese reported data (21). However, overall prevalence of HGV among the patients undergoing hemodialysis could vary from 1.3% to 55%. On the other hand, according to the reported data, concomitant infection of HGV with HCV or HBV was not similar. Even in some cases reports showed no co-infection at all (23, 24). Overall, HGV infection is more contemporaneous with HCV. This may be due to similarities in their transfection route. In the current study there was only one case of concomitant infection of HGV and HCV; but HGV and HBV co-infection was found in two cases. However, a previus study showed the frequency of chronic HBV and HCV concomitant infection with HGV as 55% and 18%, respectively (25).
Other epidemiological studies in Iran reported the prevalence of 12.6% for the patients undergoing hemodialysis in Tehran (29). The other study reported 4.8% HGV RNA positive cases among the blood donors, who were negative for HIV-Ag/Ab, HCV-Ab, and HBs-Ag (30). However, the current study reported the prevalence of 3.14% HGV RNA among the patients undergoing hemodialysis, which was different from the results obtained in other countries, but agreed with the previous studies in Iran. It is noteworthy that up to now no definitive pathogenesis has been found for HGV. However, it is necessary to detect HGV in the societies since, despite its questionable role for hepatitis and non-pathogenic feature, it may be converted to a dangerous pathogen in the future; its role may also be revealed in its healthy carriers.
The current study emphasized that transplantation may be an important way of HGV transmission in the Iranian kidney transplantation recipients; therefore, HGV should be considered routinely for kidney donors. Higher rate and chance of HCV positivity in the patients with renal failure than transplantation recipients may be due to more episodes of blood transfusion and exposure to blood transmitted HCV. Since the studies newly started considering HGV in Iran, it is necessary to continue such studies especially HGV sequencing in the patients undergoing hemodialysis and those with hepatitis B and C infections. Investigating the association of HGV and clinical manifestations of the aforementioned patient groups could be valuable for a clear concept about their disease severity and treatment. Regarding the HGV transmission through blood or blood products, screening tests for HGV appears to be necessary for blood donors.