1. Background
Helicobacter pylori is recognized as the most common cause of chronic active gastritis and is also an important pathogenic factor in peptic ulcer disease. The outcome of infection is thought to be related to severity and distribution of H. pylori-related inflammation (1). Biological factors that affect clinical outcome in H. pylori infection have been widely studied. In addition to bacterial virulence determinants in H. pylori strains, immunological factors in the host are likely to play a crucial role in different clinical expression of H. pylori-infection (2-4). H. pylori infection first induces neutrophilic gastritis, which progresses to chronic gastritis in most people (5, 6). Activation and migration of these inflammatory cells into the gastric mucosa is related to increased production of proinflammatory cytokines, including IL-8, IL-1β, IL-6, TNF-α and IL-6 which are believed to contribute to maintaining the gastric inflammation and causing epithelial cell damage (7-9). Persistent inflammation, possibly intensified via the inflammatory cytokine cascade and generation of H. pylori-specific T and B cell immune responses (2), eventually leads to gastric atrophy, hypochlorhydria and increased risk of gastric carcinoma (10). In spite of cellular and humoral immune responses, H. pylori infection shows lifelong persistence in most cases (11).
The clinical outcome of H. pylori infection is suggested to be linked to certain strains such as cytotoxin-associated gene (cagA) and vacuolating cytotoxin (vacA) (12). The cagA gene as a marker for the presence of a Pathogenicity Island (cagPAI) has been shown to be involved in induction of proinflammatory chemokine release, also associated with more severe gastritis and higher prevalence of peptic ulcer and gastric cancer (13-15). VacA has two variable parts: The s (signal peptide) region exists as allele s1 or s2. The m (middle) region exists as m1 or m2 allelic type. The allele combination s-m1 confers high, s1m2 intermediate and s2m2 low toxin activity. Most vacA s1 strains are cagA-positive, thus the two markers are closely related (16). There are geographic differences between vacA status and H. pylori-related diseases. In western countries, infection with vacA s1 strain is more common in patients with peptic ulcer than those with chronic gastritis. However in Asian populations, the association between vacA diversity and clinical outcome is not established (17, 18).
The complex interplay between the bacterium and the host immune reaction has not yet been fully understood. IL-6 is a multifunctional cytokine produced by immune and many non-immune cells and functions as an inflammatory mediator and endocrine as well as metabolic function regulator (19). However, recent data suggests that IL-23 is not the differentiation factor, TGF- β plus IL-6 induce Th17 cell development from naive T cells (20). Th17 cells produce IL-17F, IL-17 and IL-22, which are thought to be required for the control of a variety of bacterial and fungal infections at mucosal surfaces (21). IL-6 has also been implicated in the pathogenesis and/or prognosis of several different tumors, including multiple myeloma and lymphoma (22). However, the association between levels of IL-6 in mucosal gastric patients and the major virulence factors of H. pylori (cagA, especially vacA allelic variants) has not yet been clarified. In the present study, we aimed to clarify the role of mucosal IL-6 mRNA expression in human gastric patients and its correlation with various virulence factors.
2. Objectives
The current study aimed to determine mucosal IL-6 mRNA expression level and its correlation with the grade of chronic gastritis among H. pylori-infected patients with chronic gastritis from Shahrekord, Iran. The present study aimed to determine: 1) IL-6 expression in H. pylori-infected gastric mucosa, 2) The effect of virulence factors of H. pylori (cagA and vacA allelic variants) on the mucosal IL-6 mRNA level in gastric mucosa, and 3) the correlation between mucosal IL-6 mRNA levels and the grade of chronic gastritis.
3. Patients and Methods
3.1. Patients and Sampling
A total of 102 subjects, undergoing endoscopy at “Hajar university hospital” in Shahrekord, Iran, were included in the study. The study was approved by the human research ethics committee at Shahrekord university of medical sciences and informed consent was obtained from each volunteer before participation. The subjects were designated in H. pylori-positive (n = 58: 23 males, 35 females; mean age: 41.71 ± 15.41 years) and H. pylori-negative (n = 44: 20 males, 24 females; mean age: 38.14 ± 16.69 years) groups. H. pylori-infection was determined by rapid urease test, PCR (16srRNA, glmM) (3) and histological examination of biopsies taken from the corpus. Patients were classified as H. pylori-infected only if the four tests had positive results. Four biopsies were collected from 58 H. pylori-infected and used for rapid urease test, histological examination, assessment of bacterial virulence factors, detection of H. pylori and cytokine RNA analysis.
3.2. Histological Examination
Sections of biopsy specimens were embedded in 10% buffered formalin and stained with hematoxylin and eosin to examine gastritis and with Giemsa to detect H. pylori. The histological severity of gastritis was blindly graded from normal to severe based on the degree of mononuclear cell (MNC) and polymorphonuclear leukocyte (PMN) infiltration and atrophy according to the Updated Sydney system (23) on a four-point scale: 0, no; 1, mild; 2, moderate; and 3, severe changes.
3.3. PCR Amplification
DNA for polymerase chain reaction (PCR) was extracted using the Biospin Tissue Genomic DNA Extraction Kit (BioFlux, Japan). Specific primers for PCR amplification of different genes are shown in Table 1 (3). For vacA and cagA evaluation, the PCR program comprised 35 cycles of denaturation (at 94°C for 30 seconds), annealing (at 56°C for 30 seconds, extension at 72°C for 30 seconds) and one final extension (at 72°C for 5 minutes).
| Primer Designation | Primer Sequence | Size of PCR Product, bp |
|---|---|---|
| 16srRNA | 109 bp | |
| HP1: 5’-CTGGAGAGACTAAGCCCTCC-3’ | ||
| HP2: 5’-ATTACTGACGCTGATTGTGC-3’ | ||
| glmM (ureA) | 249 bp | |
| GLM MF: 5’-AAGCTTTTAGGGGTGTTAGGGGTTT-3' | ||
| GLM MR: 5’-AAGCTTACTTTCTAACACTAACGC-3' | ||
| vacA m1/m2 | ||
| vacAmF: 5’-CAATCTGTCCAATCAAGCGAG-3’ | 567 bp (m1) | |
| vacAmR: 5’-GCGTCTAAATAATTCCAAGG-3’ | 642 bp (m2) | |
| vacA s1/s2 | ||
| VA1-F: 5’-ATGGAAATACAACAAACACAC-3’ | 259 bp (s1) | |
| VA1-R: 5’-CTGCTTGAATGCGCCAAAC-3’ | 286 bp (s2) | |
| cagA | 232 bp | |
| cag1: 5’-ATGACTAACGAAACTATTGATC-3’ | ||
| cag2 5’-CAGGATTTTTGATCGCTTTATT-3’ |
PCR Primers for Amplification of Virulence Factors
3.4. Quantitative Analysis for IL-6 mRNA in the Gastric Mucosa Using Real-Time PCR
Total RNA was isolated from whole gastric biopsy specimens using total RNA extraction biozol (bioflux, Japan). An aliquot containing 0.2 μg of total RNA was used for reverse transcription reaction, which was conducted using the superscript first-strand cDNA synthesis system (Fermentas, Finland) according to the manufacturer’s instructions. The sequences of oligonucleotide primer and probe are shown in Table 2 (3, 24). The quantification of IL-6 mRNA levels was performed using a Rotor-Gene 3000 (Corbett, Australia). Real-time PCR reactions were performed in a total volume of 25 μL containing 3 μL of synthesized cDNA solution, 12.5 μL of 2x Rotor-gene probe PCR master mix (Qiagen, Germany), 500 nM of each primer and 250 nM of the TaqMan probe. Amplification program included a pre-warming step (10 minutes at 94°C), denaturation step (94°C for 15 seconds) and an annealing/extension step (60°C for 60 seconds). The expression levels of cytokine mRNA were expressed as the ratio of cytokine mRNA to β-actin mRNA (cytokine mRNA/β-actin mRNA). Each assay was performed in duplicate and each cytokine assay was performed testing all RNA samples in the same experiment. Relative quantification of cytokine to β-actin (cytokine mRNA/β-actin mRNA) was determined using the 2−ΔCt = 2− (Ct, IL-6− Ct, β-actin) method.
| Gene | Primer and Probe Sequence |
|---|---|
| β-actin | |
| Forward 5-AGCCTCGCCTTTGCCGA-3 | |
| Reverse 5-CTGGTGCCTGGGGCG-3 | |
| Probe FAM-CCGCCGCCCGTCCACACCCGCC-TAMRA | |
| IL-6 | |
| Forward 5- GGTACATCCTCGACGGCATCT-3 | |
| Reverse 5- GTGCCTCTTTGCTGCTTTCAC-3 | |
| Probe FAM-TGTTACTCTTGTTACATGTCTCCTTTCTCAGGGCT-TAMRA |
Primer and Probe Sequences Used in This Study
3.5. Statistical Analysis
Data was analyzed using GraphPad Prism 5 Demo (GraphPad software, San Diego, California, USA). Age in the two groups was compared by unpaired student’s t-test. In statistics, normality tests were used to determine whether a data set was well-modeled by a normal distribution. Cytokine expression was presented as mean and differences between infected and uninfected groups were analyzed using student’s t-test. Also for gene expression, values between H. pylori-infected patients and virulence factors parametric student’s t-test, and for comparison of more than two groups, one-way ANOVA test were used. P values < 0.05 were considered as statistically significant.
4. Results
4.1. Detection of H. pylori in Gastric Mucosa of Patients by PCR
We evaluated two primer pairs for the detection of DNA H. pylori in gastric mucosa of patients (Figures 1 and 2).
4.2. IL-6 Gene Expression Enhanced in H. pylori-Infected Gastric Mucosa
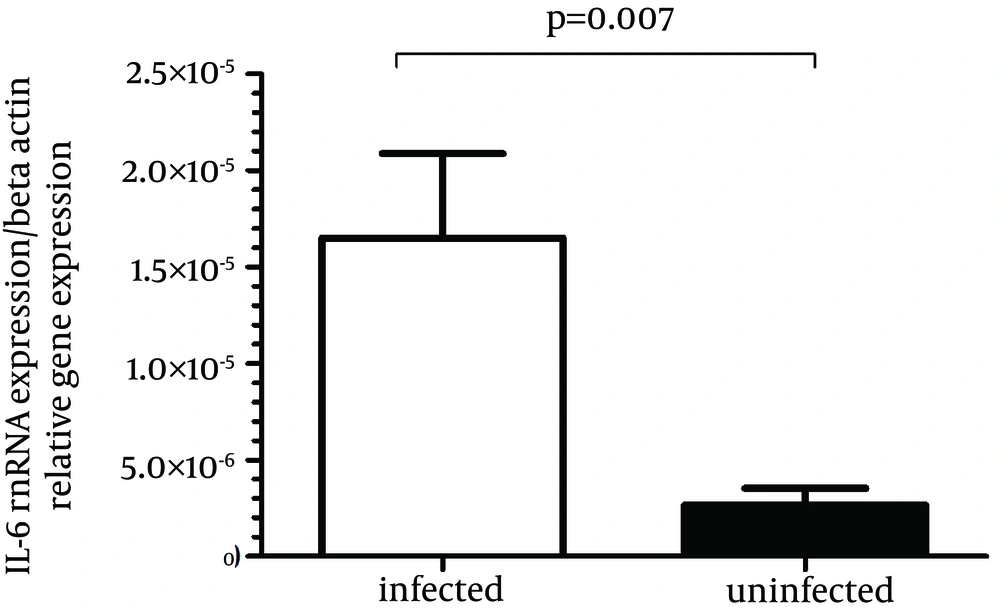
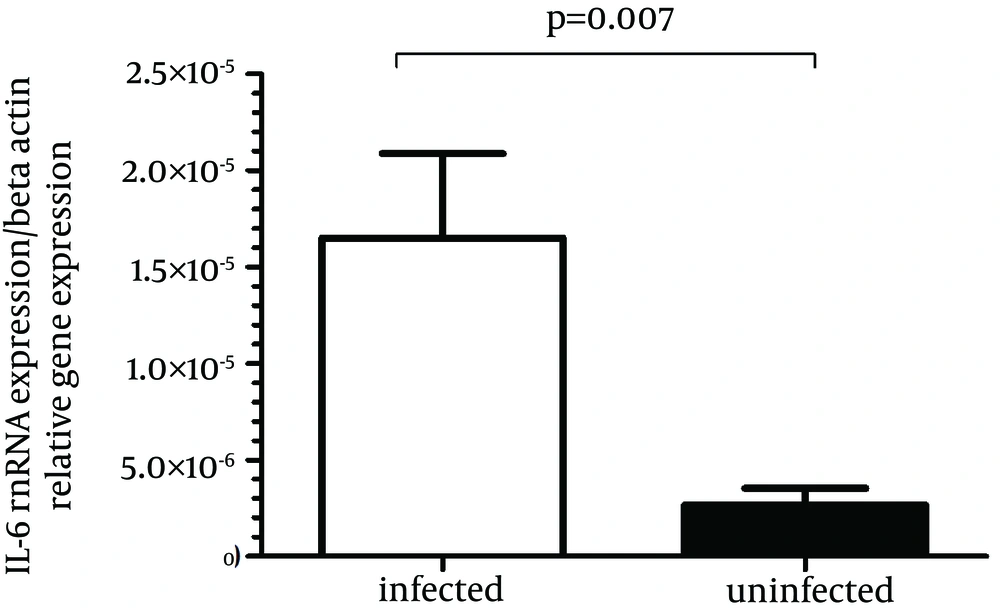
IL-6 gene expression was significantly more in biopsy specimens of H. pylori-infected patients compared to H. pylori-negative subjects (P = 0.007) (Figure 3). The mean H. pylori-positive group was 0.000016 and the mean H. pylori-negative group was 0.0000026, then the difference in the IL-6 mRNA expression in the H. pylori-positive group compared with the H. pylori-negative group would be 0.000016/0.0000026 or 6.2-fold.
4.3. Effect of vacA Allelic Variants in H. pylori-Infected on the Mucosal IL-6 mRNA Level in Gastric Mucosa
Our results showed that in patients with H. pylori infection, mucosal IL-6 mRNA level was independent from the vacA status. The difference in the IL-6 mRNA expression in the vacA s1-positive H. pylori strains compared with the vacA s2-positive H. pylori strains was 0.000018/0.0000043 or 4.2-fold (P = 0.053). Mucosal IL-6 mRNA expression in H. pylori-infected patients with vacA m2-positive was not significantly higher than those observed in H. pylori-infected patients with vacA m1-positive (0.0000187/0.000012 or 1.5-fold) (P = 0.534). Also mucosal IL-6 mRNA expression in gastritis patients with vacA s1m1-positive was not significantly higher than those observed in gastritis patients with vacA s1m2-positive (0.000012/0.000022 or 0.53-fold) and vacA s2m2-positive (0.000012/0.0000043 or 2.76-fold) (P = 0.157).
4.4. Effect of cagA Virulence Factor in Patients With H. pylori Infection on the Mucosal IL-6 mRNA Level in Gastric Mucosa
The mucosal IL-6 mRNA level was independent of cagA virulence factor status. The mean H. pylori-positive group with cagA was 0.000021 and the mean H. pylori-positive group without cagA was 0.000007, then the difference in the IL-6 mRNA expression in the cagA-positive H. pylori strains compared with the cagA-negative H. pylori strains would be 0.000021/0.000007 or 3.1-fold (P = 0.107).
4.5. Correlation Between Mucosal IL-6 mRNA Levels and the Grade of Chronic Gastritis
Figure 4 shows the association between mucosal IL-6 mRNA expression and chronic inflammation scores in patients with H. pylori infection. There was a significant correlation between mucosal IL-6 mRNA expression and the grade of chronic gastritis (P = 0.014). The degree of chronic inflammation was assessed and graded as follows; (25) 43.1% mild, (20) 34.48% moderate and (13) 22.41% severe. Mucosal IL-6 mRNA expression in gastritis patients with severe grades was significantly higher than those observed in gastritis patients with moderate (0.000048/0.0000239 or 2.08-fold) and mild grades (0.000048/0.0000065 or 7.38-fold).
5. Discussion
Our findings showed that the mucosal IL-6 mRNA level increased in patients with H. pylori infection compared with uninfected patients and correlated positively with the grade of chronic gastritis in patients with H. pylori infection. In addition, mucosal IL-6 mRNA expression was independent from the virulence factors of H. pylori. A previous study showed that the mucosal levels of IL-1β, IL-6 and IL-8 were increased in the gastric mucosa compared with uninfected persons (25). These findings indicated the association between these cytokine levels and H. pylori infection status. A high degree of polymorphonuclear infiltration for long periods of time may be a risk factor of carcinogenesis, as the oxidative burst produced by disintegration of cells in the gastric mucosa liberates substances with mutagenic potential (26).
CagA and vacA are the major virulence factors of H. pylori. Persistent infection by H. pylori enables these toxins (cagA and vacA) to stimulate gastric epithelial cells to produce a large number of cytokines such as TNF-α and IL-1, IL-6 and IL-8, thus generating an inflammatory reaction (27). Results of Yamaoka et al. in patients with H. pylori infection showed that mucosal levels of IL-6, IL-7, IL-8, IL-10 and TNF-α mRNA were significantly higher in H. pylori-positive than H. pylori-negative patients, but only the level of IL-8 mRNA expression was significantly higher in cagA-positive than cagA-negative specimens (28). Serum levels of IL-6 were also higher among patients with gastric cancer compared with those with benign gastric lesions caused by H. pylori infections (29). Mucosal IL-6 levels were also higher in early gastric cancer with active H. pylori infection than without H. pylori infection, and the levels decreased dramatically after eradication of infection (25).
IL-6 has an important role as a prognostic factor for advanced gastric cancer and lymph node metastasis (30). Study of Lu et al. in gastric epithelial cells infected with H. pylori indicated that H. pylori infection induced IL-6 mRNA expression and IL-6 protein production, which was further enhanced by the cagA and OipA (31). Odenbreit et al. demonstrated that macrophages might be a major source of IL-6 in the gastric mucosa upon infection. The IL-6 induction by H. pylori in these cells is a multifactorial process, which requires the uptake and presumably degradation of H. pylori bacteria (32). It has also been demonstrated that H. pylori heat shock protein sixty (HSP60) plays a role in the pathophysiology of H. pylori gastritis through the release of IL-6 by macrophages. This finding may support this hypothesis that the host is capable of recognizing and responding to a H. pylori protein that is synthesized and/or is essential for the bacterial survival and should be present in all of the H. pylori strains. In fact, recognition of H. pylori HSP60 by non-specific immunity might represent a mechanism by which H. pylori would be able to induce chronic gastritis, which is an ordinary feature of all infections attributed to this pathogen (33). Moreover, H. pylori urease is a potent activator of human mucosal macrophages for IL-6 generation (34).
The results above show that in addition to bacterial virulence factors, other compounds of H. pylori such as urease and HSP60 may be involved in induction of IL-6 production. In conclusion, in H. pylori-infected patients, IL-6 is originally found as a B lymphocyte stimulatory factor-2, which induces activated B lymphocytes into antibody production (35, 36). IL-6 combined with TGF-β preferentially induces the differentiation of naive CD4 positive T cells into Th17 cells; whereas, IL-6 inhibits TGF-β induced regulatory T cell (Treg) development (37, 38). As a consequence, Th17/Treg imbalance may cause the onset and progression of autoimmune and chronic inflammatory diseases (39, 40). In summary, dysregulated persistent IL-6 production has been implicated in the development of various chronic inflammatory autoimmune diseases. In summary, the present study confirmed the findings of some earlier investigations indicating that dysregulated persistent IL-6 production may have a role in the pathogenesis of H. pylori-associated gastritis.