1. Background
Toxoplasma gondii is a widespread, obligate and intracellular protozoan parasite belonging to the phylum Apicomplexa that infects any nucleated cell of a wide range of vertebrate animals, including mammalian and avian species and causes the most common zoonotic infection in humans (1, 2). The only known definitive hosts for this organism are cats and other felines while all nonfeline vertebrates including humans, domestic and wild livestock, birds and marine mammals act as intermediate hosts of this protozoan (3, 4). Humans become infected postnatal horizontally by ingestion or handling of undercooked or raw meat containing viable tissue cysts, consuming food (fruits and vegetables) and water contaminated with sporulated oocysts or drinking of unpasteurized milk of infected animals.
Humans could be infected accidentally by ingesting oocysts from the environment with exposure to contaminated soil (gardening without gloves) or cat litter, organ transplantation or blood transfusion from infected donors and laboratory accidents. Another way of infection is by tachyzoites that are passed to the fetus via the placenta when an uninfected mother acquires infection during pregnancy (5-8). Toxoplasmosis was estimated to infect about one-third of world’s human population, but its prevalence is affected by the strain of parasite (and genotype), age, geographical situation, occupational groups, food habits, social culture and thus varies from less than 10% - 90% in different countries (9, 10).
Toxoplasmosis in healthy adult humans is usually asymptomatic chronic form or associated with self-limited symptoms; therefore, often goes unnoticed and rarely needs treatment (11). Meanwhile, immunocompromised patients and pregnant women who acquire their infection during gestation, fetuses and newborns who are congenitally infected and those with chorioretinitis are the serious examples of toxoplasmosis. However, immunocompromised individuals, such as patients with AIDS and persons undergoing therapies for malignancies, transplants or lymphoproliferative disorders because of reactivation of a latent infection may present severe forms of the disease, such as encephalitis and pneumonitis (12, 13). Another risk group consists of children infected during pregnancy, depending on the gestational age at which the pregnant woman acquires the infection, the consequences may vary from death of the fetus in utero and spontaneous abortion to hydrocephalus, microcephaly, retinochoroiditis in the surviving infants (14, 15). Moreover, the parasite causes economic losses to the livestock industry mainly among food producing animals as the infection may lead to embryonic death, abortion, mummification, still birth and neonatal loss in pregnant animals especially sheep, goats and pigs, as well as a source of transmission to humans (16). Thus, toxoplasmosis continues to be a real problem in public health and livestock husbandry.
The primary strategy for the treatment of toxoplasmosis is chemical drugs; however, they are poorly tolerated, have side effects, drug-resistance and are not an effective treatment for tissue cysts of T. gondii in humans and animals also they cannot prevent reinfection (17). Therefore, the development of an effective and safe vaccine against T. gondii infection is an important, urgent goal and appears to be achievable. Some antigens of T. gondii are relatively effective candidates for DNA vaccine includes Surface Antigen Glycoproteins (SAGs), Excretory-Secretory Dense Granule Proteins (GRAs), Rhoptry Proteins (ROPs), and Micronemal Proteins (MICs), which were tested in recent years (17-19). Surface antigen 1 (SAG1) is one of the SAGs of T. gondii that anchors to the cell membrane of this protozoan by a glycosylphosphatidylinositol anchor and plays an important role in the processes of host cell attachment, penetration and host immune evasion (20-22).
Although this protein only accounts for 3% - 5% of total proteins of this parasite it is the most abundant SAGs in the tachyzoites of T. gondii and during toxoplasmosis, the majority of the antibodies are reactive against it. Surface antigen 1 is a hydrophobic 30 kDa protein with an acidic pH that has been identified in the parasitophorous vacuole membrane and in the intravacuolar membranous network. The gene of SAG1 has a single-copy with a length of 1.1 kb that does not have introns in the coding region or a TATA box in the promoter region (19, 23). In the present study, we cloned and expressed TgSAG1 from RH strain of T. gondii and analyzed its gene expression in Chinese Hamster Ovary (CHO) cells.
2. Objectives
The purpose of this study was to clone SAG1 gene from the genotype 1 of T. gondii, RH strain in eukaryotic expression vector pVAX1. Moreover, the major objective of the present study was to express SAG1 using the eukaryotic expression vector pVAX1 in eukaryotic cells to evaluate the high-level recombinant protein production use for DNA vaccine in the future.
3. Materials and Methods
The polymerase chain reaction (PCR) cloning Kit was purchased from 5prime (Germany) and other kits were purchased from Qiagen (Germany), restriction endonucleases were purchased from New England Biolabs (New England Biolabs Inc., USA). T4 DNA ligase, Taq polymerase, dNTP and protein weight markers were purchased from Roche (Germany). 5-Bromo-4-chloro-3-indolyl β-D-galactopyranoside (X-gal) and Isopropyl-β-D-thiogalactopyranoside (IPTG) were purchased from Sigma-Aldrich Corporation (Germany). The Escherichia coli strain DH5α and pVAX1 expression vector were purchased from Novagen (Novagen Inc., Madison, WI, USA). All chemicals were obtained from Merck (Germany), Sigma-Aldrich (Germany) and HiMedia (India) Corporations.
3.1. Parasite
The tachyzoites of the T. gondii RH strain were inoculated in peritoneal cavity of BALB/c mice. After 3 - 4 days, the parasites were collected, washed and suspended in Phosphate Buffer Saline (PBS, pH 7.2) for lysate preparation and DNA extraction (24).
3.2. Gene Amplification of Surface Antigen 1
Genomic DNA of T. gondii RH strain was extracted using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. A pair of primers based on the full protein-coding region of the T. gondii SAG1 gene sequence (Accession number GQ253075) was designed by the DNASIS version 2.6 primer designer software with NheI and XhoI restriction sites at 5′ end of forward and reverse primers, respectively. The sequences were SAG1F: 5′-AAGCTAGCCACCATGTCGGTTTCGCTGCACC-3′ and SAG1R: 5′-AGGAATTCTCGAGCTCACGCGACACAAGCTGC-3′ (Bioneer Corporation, South Korea). The simple Kozak translational consensus sequence was introduced in the beginning of the PCR product by its addition to the forward primer. Also, the PCR reaction was performed in a total volume of 50 μL using 100 ng DNA, 1 μL forward and reverse primers at 25 pmol, 50 mМ Mgcl2, 200 μМ d NTP, 10X PCR buffer, and 2.5 u Taq polymerase (high fidelity, Roche, Mannheim, Germany).
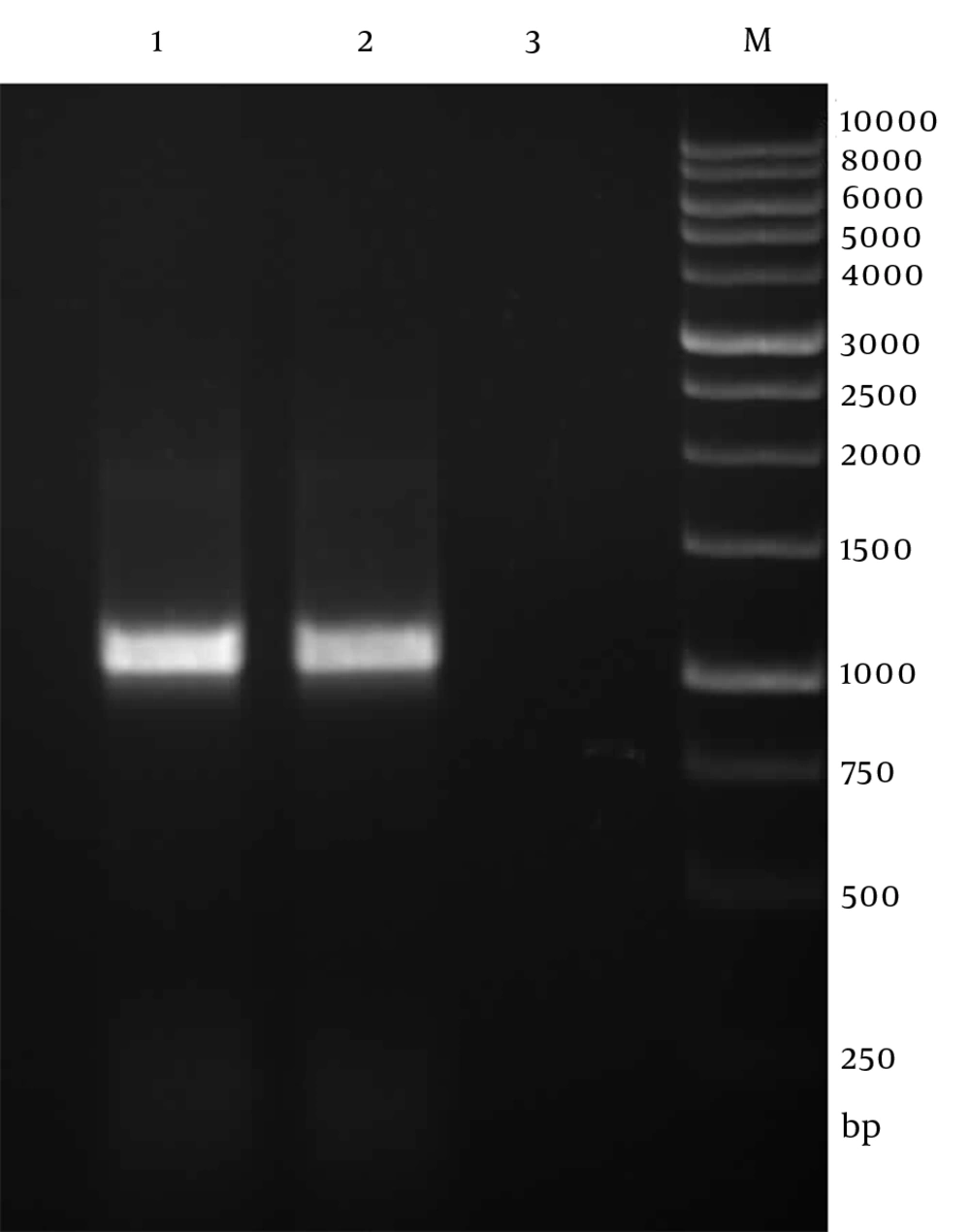
The PCR condition was as follows: predenaturation at 94°C for 5 minutes; denaturation at 94°C for 1 minute, annealing at 60°C for 30 seconds and extension at 72°C for 70 seconds, followed by 30 cycles; final extension at 72°C for 10 minutes in a thermal cycler (Bio-Rad, Hercules, CA, US). The PCR product was analyzed by electrophoresis on 1% agarose gel in 1X TBE buffer and visualized using the ethidium bromide staining on UV transilluminator. The PCR product was purified from the agarose gel using QIAquick gel extraction kit (Qiagen, Hilden, Germany) according to the manufacturer’s recommendation and DNA concentration was measured by spectrometry at OD260 and OD280 using Nanodrop 2000 (Thermo Scientific, USA).
3.3. Cloning of SAG1 Gene
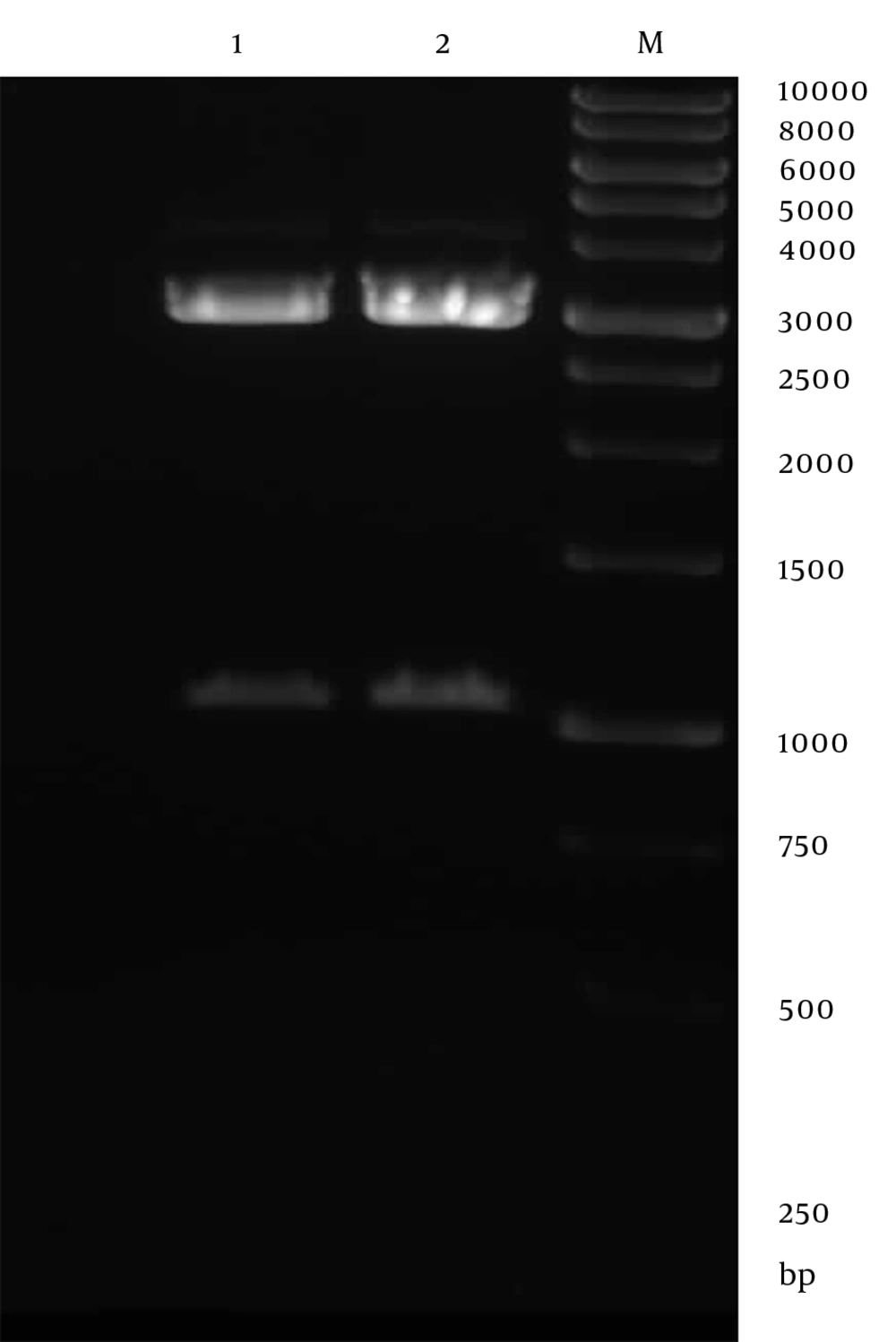
The PCR product was cloned into the pPrime vector via T/A cloning method using the 5 prime perfect PCR cloning Kit (5prime, Hamburg, Germany) according to the manufacturer’s instructions. Then the pPrime-SAG1 was transformed in calcium chloride E. coli DH5α competent cells (Novagen Inc., Madison, Wis) and dispensed on Luria-Bertani (LB) medium (HiMedia, Mumbai, India) agar plates containing kanamycin (50 mg/L). Bacterial colonies were screened by agar plate containing X-gal (Sigma, USA) and IPTG (Sigma, USA) to discriminate between recombinant (white) and no recombinant (blue) plasmid containing ones (25). Several suspected colonies (white) were further analyzed by colony PCR. After selecting recombinant clones, the plasmid DNA was extracted from cells cultured overnight with shaking at 250 rpm at 37°C by using the Miniprep plasmid purification kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. The selected recombinant plasmids were verified by restriction digestion with NheI and XhoI restriction enzymes (New England BioLabs, Ipswich, MA, USA) and sequenced using M13 primers.
3.4. Subcloning of Surface Antigen 1 Gene
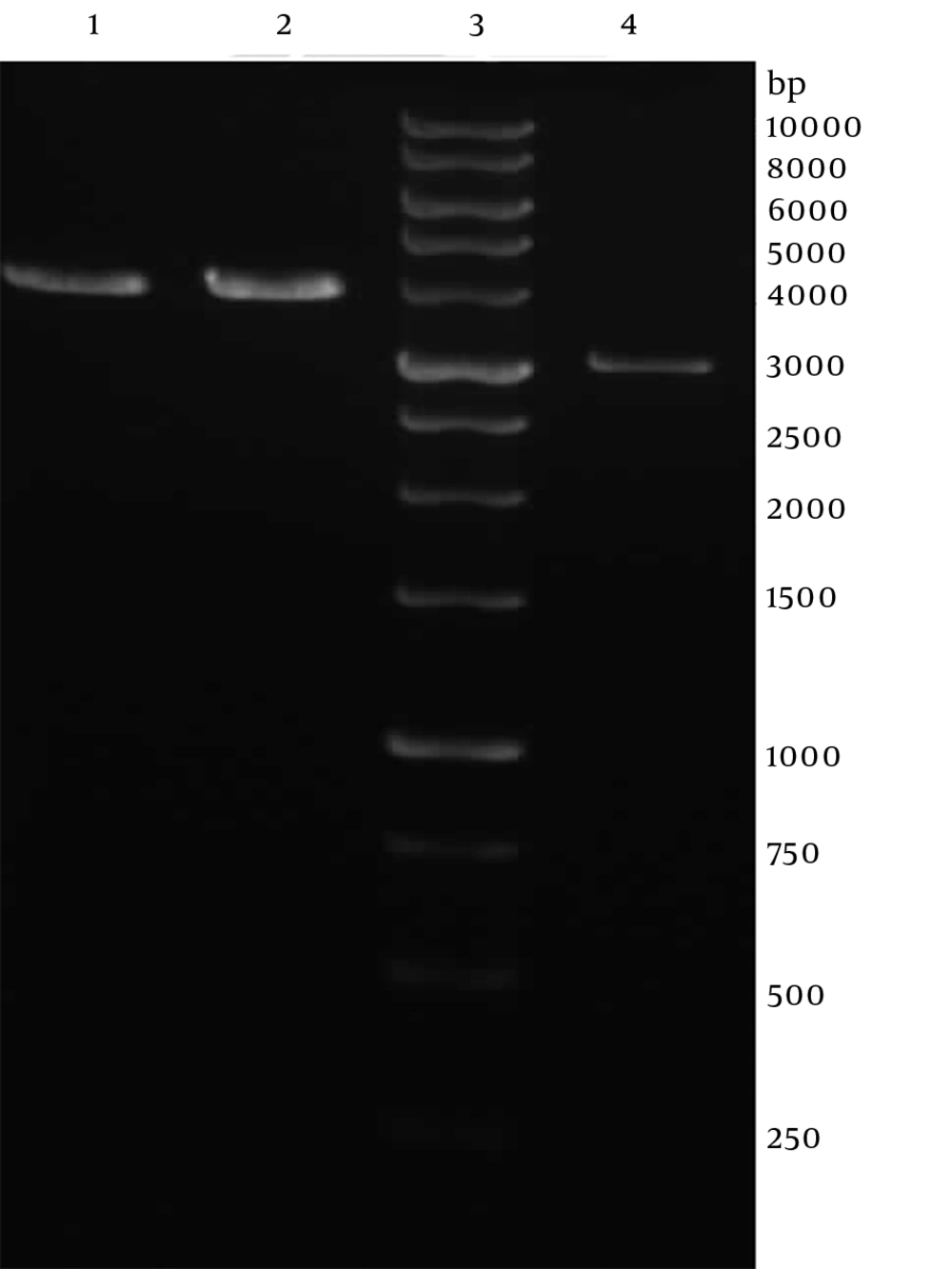
The pPrime vector carrying the SAG1 gene and the eukaryotic expression vector pVAX1 (Invitrogen, Carlsbad, USA) were digested by NdeI and XhoI restriction enzymes. After thermal inactivation of the restriction enzymes and analysis by LMP agarose gel electrophoresis, the linearized pVAX1 plasmid and the SAG1 gene were extracted from the %1 agarose gel by agarose gel DNA extraction kit (Qiagen, Hilden, Germany). The purified linear vector and insert were subjected to ligation reaction using T4 DNA ligase (Roche, Mannheim, Germany). E. coli DH5α competent cells were transformed with 2 uL of ligation product. Transformed E. coli cells were selected on LB medium agar plates containing kanamycin (50 mg/L). Several colonies were assayed by colony PCR using specific primers (SAG1F and SAG1R). After selecting recombinant clones, the plasmid DNA was extracted from the cells cultured overnight by using the Miniprep plasmid isolation kit (Qiagen, Hilden, Germany) and confirmed by PCR, restriction-enzyme digestion, followed by DNA sequencing using T7 and BGH primers.
3.5. Expression of pVAX-Surface Antigen 1 Plasmid in Chinese Hamster Ovary CHO cells
3.5.1. Transient Transfection
Chinese Hamster Ovary (CHO) cell line, were maintained in our laboratory and routinely cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with penicillin (100 IU/mL), streptomycin (100 mg/mL) and 10% Fetal Bovine Serum (FBS) at 37°C with 5% CO2 atmosphere. One-day before transfection, 1 – 3 × 104 CHO cells were seeded in 6-well plates (35-mm wells). The recombinant pVAX1-SAG1 plasmid was transfected into cells with X-tremeGENE9 DNA transfection reagent (Roche) according to the manufacturer’s instructions. In this way, 3μL X-tremeGENE9 DNA transfection reagent and 100 μL serum-free medium were mixed and incubated for 5 minutes at room temperature and 1 μg DNA was added to the mixture and incubated 20 minutes after it was added to a 6-well plate at 70% - 90% confluency and plates were incubated for additional 48 hours at 37°C in 5% CO2, 95% humidity. Chinese hamster ovary transfected with pVAX1 without insert and un-transfected cells were used as controls. Then, SAG1 gene expression in transfected cells was evaluated by RT-PCR, immunofluorescence assay and western blot analysis.
3.5.2. Reverse Transcriptase Polymerase Chain Reaction Assay (RT-PCR)
The transfected cells and the control cells were rinsed with ice-cold PBS for three times and RNA was extracted using the Trizol reagent (Invitrogen) according to the manufacturer’s instructions. DNase I digestion was also performed to minimize the possibility of genomic DNA contamination. The extracted total RNA (1 μg) was employed for first-strand cDNA synthesis using 20 μL of AccuPower RT-Premix (Bioneer, Korea) at 55°C for 1 hour and 95°C for 5 minutes. cDNA from the reverse transcription reaction was then used in the subsequent PCR reactions with SAG1F and SAG1R primers.
3.5.3. Immunofluorescence Assay
The SAG1 expression in the cells was detected using an indirect immunofluorescence assay (IFA). In brief, the cells were fixed with cooled-acetone, and washed with PBS-0.1% Triton-X-100 (PBS-T) for three times. The cells were incubated with 1:100 diluted mouse anti-T. gondii p30 monoclonal antibody (Abcam, USA) diluted at room temperature for 60 minutes and washed three times by PBS-T, followed by incubation in 1:300 diluted FITC-labeled goat antimouse IgG antibody (Santa Cruz Biotechnology, Inc., USA) at room temperature for 60 minutes. After washing three times with PBS-T, incubation was performed in dark with DAPI (4', 6-Diamidino-2-phenylindole) at RT for 5 minutes and monolayers were covered with glycerine and examined for specific fluorescence under an Olympus IX70 multi-parameter fluorescence microscope (Olympus, Tokyo, Japan) (26). Photographs were taken at the magnification × 100 using a canon camera.
3.5.4. SDS-PAGE Sodium Dodecyl Sulfate-Poly-acrylamide Gel Electrophoresis and Western Blot
The transfected and untransfected cells were washed with ice-cold PBS and collected on ice with 200 μL RIPA lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% Sodium deoxycholate, 0.1% SDS) containing protease inhibitor cocktail (p2714, Sigma, USA). The cells were then exposed to freeze-thaw cycles. Then the cell lysates were boiled in the loading buffer for 10 minutes and separated using a 12% (w/v) Sodium dodecyl Sulfate-Poly-acrylamide Gel Electrophoresis (SDS-PAGE) according to the standard protocol (27). Following electrophoresis, separated proteins were transferred to Poly-vinylidene Fluoride (PVDF) membrane (Roche Diagnostics GmbH) using a Mini trans-blot electrophoretic transfer cell (Bio-Rad, Hercules, CA, USA) in transfer buffer containing 25 mM Tris (pH = 8.3), 192 mM glycine and 20% methanol at 55v for 1 hour at 4°C (28). The membrane was blocked with 5% (w/v) skimmed milk diluted in Tris-buffered saline with Tween 20 (PBS-T) (0.5 M NaCl, 0.02 M Tris pH = 8.5, 0.05% Tween 20) for 1 hour at room temperature. The membrane was furthermore incubated with the mouse anti-SAG1 antibody (dilution 1:50) at 37°C for 3 hour, followed by three times washing in TBS-T. Then the membrane was incubated with diluted goat antimouse IgG Horseradish Peroxidase (HRP)-labeled secondary antibody (1:10,000; Abcam, USA) for 1 h. The blot was then washed three times with TBS-T and visualized with ECL (Enhanced Chemiluminescence reagents, Amersham, UK) by CCD camera (Bio-Rad, USA).
4. Results
4.1. Cloning and Sequencing of Surface Antigen 1
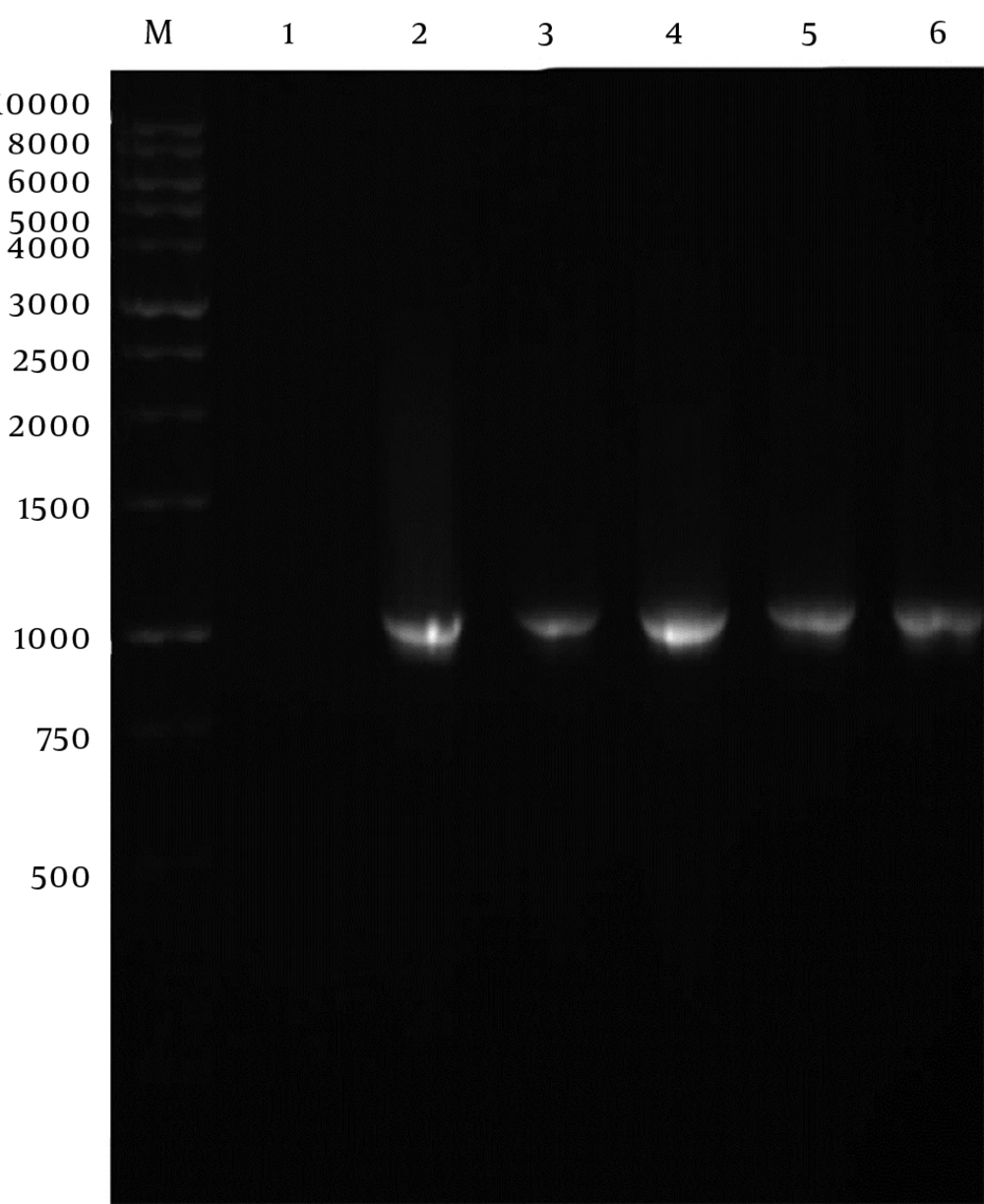
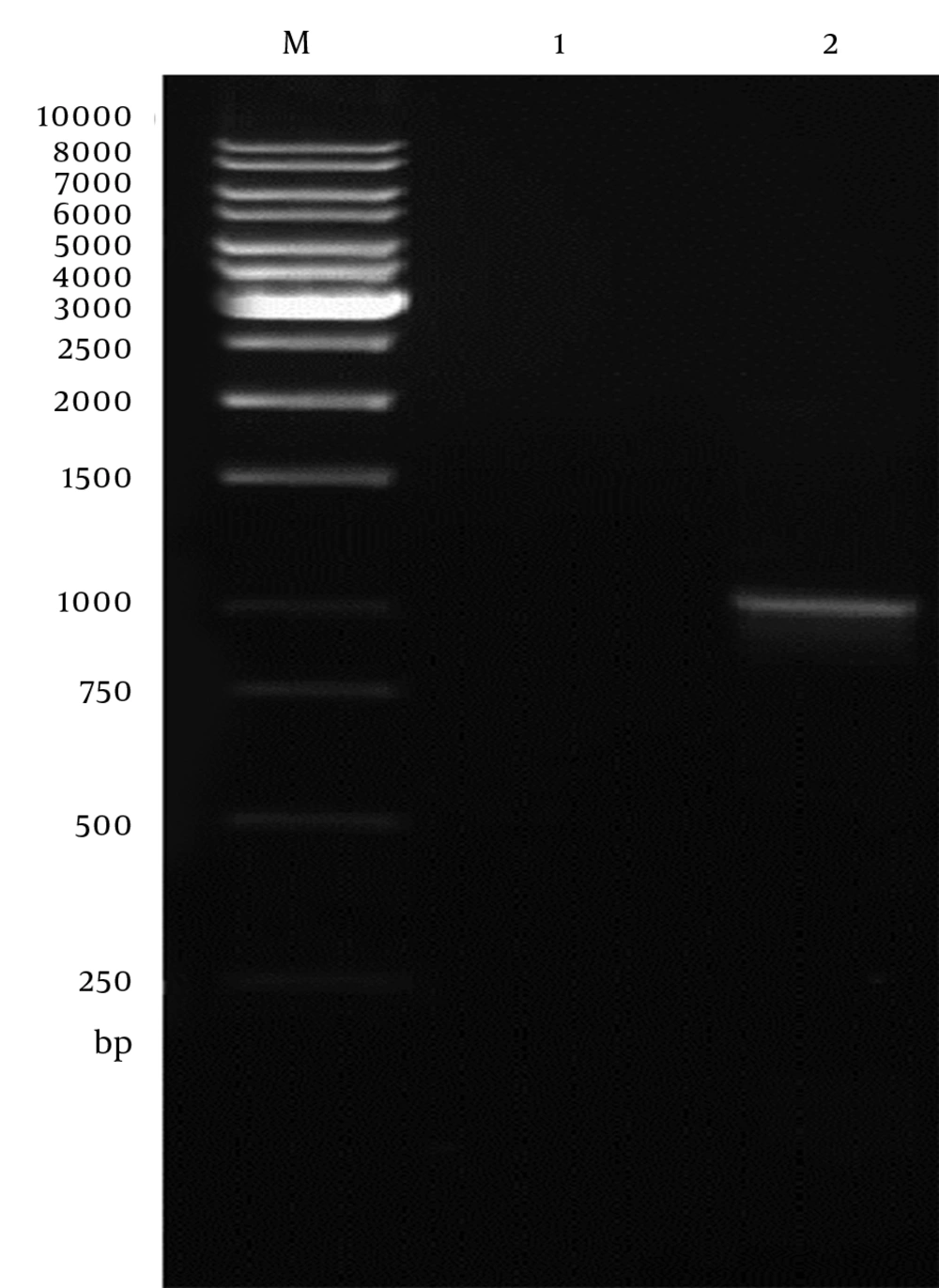
Genomic DNA of Toxoplasma tachyzoites was extracted from infected mouse peritoneum and its SAG1 gene was amplified using specific primers, showing a band about 1020 bp on agarose gel (Figure 1). The purified PCR product (1020 bp fragment) was first cloned into a pPrime vector and then subcloned into a pVax1. Following, transformation and plating of the bacterial cells on LB agar containing the selective antibiotic, IPTG and X-Gal, the presence of white and blue colonies on LB agar plates containing IPTG and X-Gal was an indication of successful transformation. Several transformed colonies were analyzed by colony PCR with the SAG1 gene specific primers. The selected plasmids (called pPrime-SAG1) were verified by restriction digestion. Sequence analysis of the isolated SAG1 gene with reported genes from and plasmid also confirmed that there were no amplification errors in the cloned SAG1 gene sequence. The sequencing result was also confirmed through comparing against databases and applying Basic Local Alignment Search Tool (Blast) software. After the confirmation of a recombinant pPrime-SAG1 plasmid, the recombinant plasmid was isolated and subcloned into a pVAX1 plasmid. After transformation of E. coli DH5α, recombinant plasmids were analyzed using the colony PCR and PCR on the isolated plasmids (Figure 2), restriction digestion (Figure 3 and 4) and sequencing (Figure 5).
4.2. Expression of pVAX1-Surface Antigene 1 Plasmid in Chinese Hamster Ovary Cells
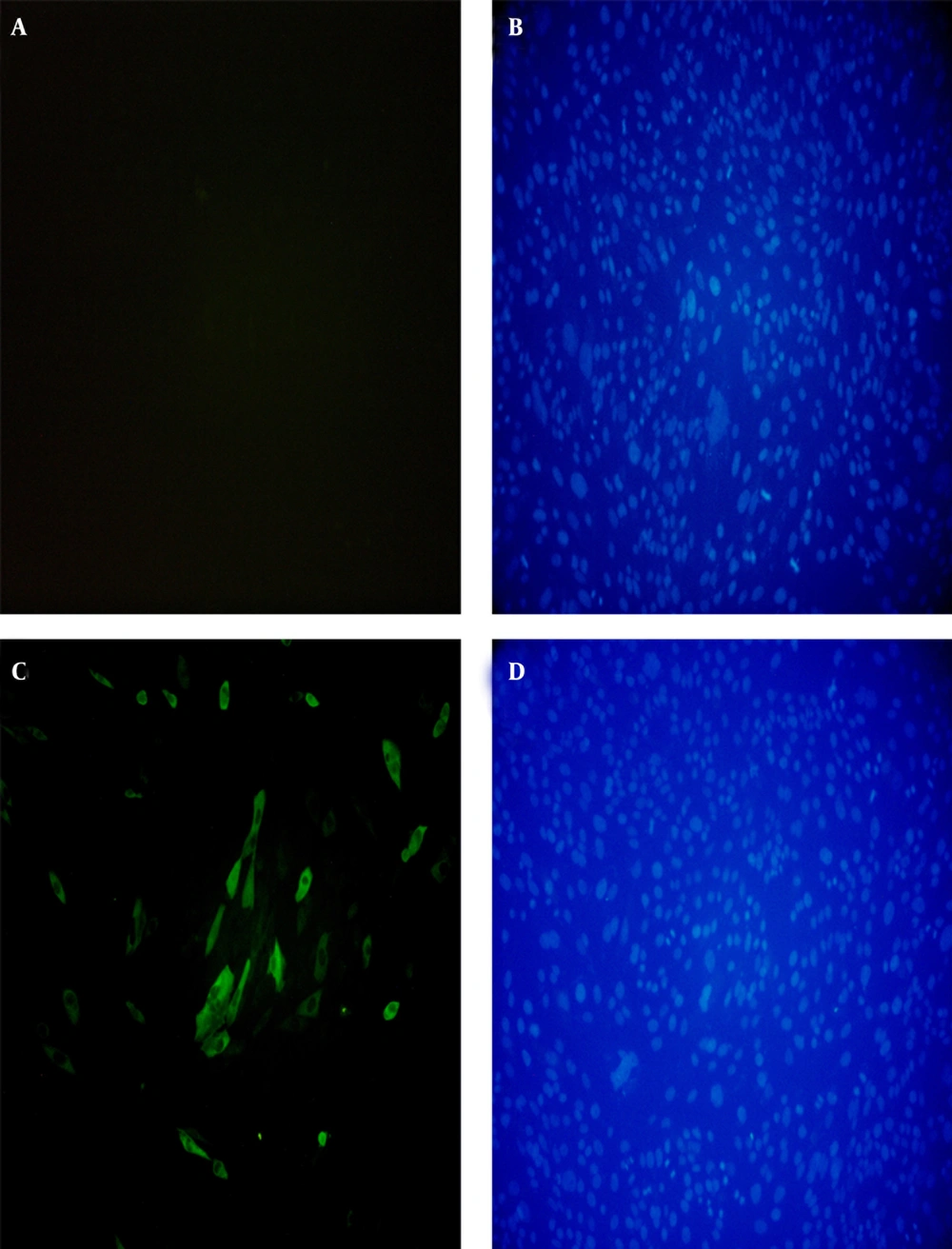
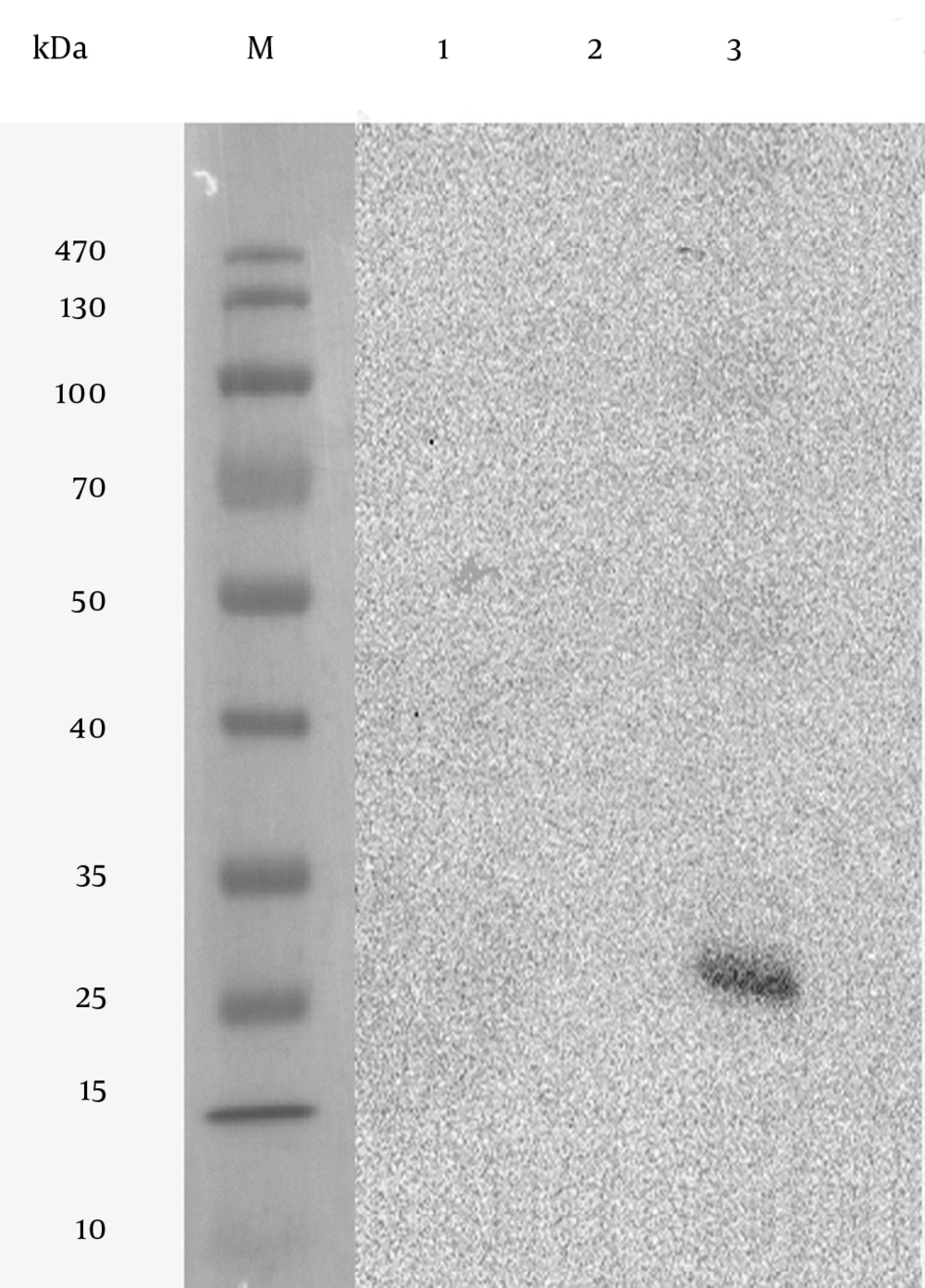
In vitro expression of the pVAX1-SAG1 was evaluated by RT-PCR, IFA and western blot at 48 hours posttransfection. Reverse Transcriptase Polymerase Chain Reaction Assay (RT-PCR) was used to determine the expression of the SAG1 gene in transduced CHO cell line; therefore, the amplified PCR fragment of 1020 bp on transfected cells was electrophoresed on agarose gel and visualized by the ethidium bromide staining that indicated the expression of the SAG1 in eukaryotic cells (Figure 6). As shown in Figure 7, the specific green fluorescence was observed in CHO cells transfected with pVAX1-SAG1, indicated expression of SAG1 protein located in cytoplasm, but not in the negative control cells transfected with the same amount of empty pVAX1 (Figure 7). Further analysis of CHO cell lysates transfected with pVAX1-SAG1 and empty pVAX1 on 12% SDS-PAGE and western blotting showed that the CHO cells were efficiently transfected and recombinant SAG1 was detected as a 30 kDa band equivalent to the native protein. In contrast, no immunoreactive material was found in the lysate of untransfected CHO cells and transfected cells with empty pVAX1 plasmid (Figure 8).
5. Discussion
Toxoplasmosis is one of the most important infectious diseases in immunocompromised human and nonimmune pregnant woman (12, 15). In veterinary, it causes tremendous problems for livestock husbandry including abortion and stillbirth in pregnant animals. Therefore, prompt diagnosis, effective treatment and prevention of this parasite is necessary to minimize the harm of this infection in humans and animals (23). Because of diagnostic problems and ineffective therapeutic approaches, vaccine development might be one of the important ways for prevention of toxoplasmosis. Thus, many studies investigated immune-prophylaxis by different immunogenic components of T. gondii (18, 19). Among them, DNA vaccination is a novel strategy in immunization field which encoding antigenic proteins of pathogens by plasmid DNAs that induced efficiency humoral and cellular immunities against a wide spectrum of infectious and noninfectious agents (29).
Among antigenic components of T. gondii, SAG1 is a major surface antigen, which was identified on the surface membrane of this protozoa using a monoclonal antibody by Handman et al. in 1980 (30). This protein is a crucial ligand for attachment and promoting invasion of tachyzoites to host cells and is able to induce both effective and durable humoral and cellular immune responses. Thus, SAG1 is a protein with an excellent antigenicity and immunogenicity to develop effective diagnosis tests or vaccines (23, 31). Additionally, among different strains of T. gondii, the sequence of SAG1 is highly conserved and share a high degree of homology between type I (pathogenic and lethal to mice) and type II/III (cystogenic) strains (32). Therefore, in recent years many researchers have tried to express SAG1 in different hosts including E. coli, Pichia pastoris, baculovirus and insect cell in order to use recombinant protein and DNA vaccine of SAG1 in experimental animals and or use it in diagnosis in serological methods (18, 19).
The recombinant SAG1 produced in E. coli often loses its specific immunogenicity; therefore, it was necessary to refold this protein or use the truncated SAG1 in serological methods (33). Kazemi et al. cloned a 957 bp fragment of SAG1 of RH T. gondii and studied its expression in E. coli to use this antigen for diagnosis of chronic and acute toxoplasmosis using using Enzyme Linked Immunosorbant Assay (ELISA) (34). khanaliha et al. used this antigen in combination of SAG2 and SAG3 for serodiagnosis of toxoplasmosis in human (35). We cloned a 1020 bp of SAG1 of T. gondii in the pVAX vector and transfect CHO cells. In other study, Solhjoo et al. cloned a 960 bp fragment of SAG1 of T. gondii into a pTZ57R cloning vector then transformed it in a E.coli TG1 strain (36). Makioka et al. cloned SAG1 gene of T. gondii into several vectors, including pTV118N, pKK233-2 and pGEX-1 then transferred it to several E. coli strains (JM109, JM105, etc.) to investigate the expression of this protein. Their results indicate that among these combinations, the pGEX-1 recombinant plasmid achieved a high expression level using a Glutathione S-transferase(GST)-fusion protein and this protein could be able to activate macrophages in animal experiments and to exhibit toxoplasmacidal effects in vitro (37).
Nielsen et al. was cloned SAG1 gene of T. gondii in frame with a sequence encoding a synthetic mimic of the tPA signal into a eukaryotic expression vector and evaluated immunization of this plasmid in C3H (H-2k) and BALB/c (H-2D) mice (38). In another survey, Solhjoo et al. (36) cloned a 960 bp fragment of T. gondii SAG1 gene into a pTZ57R/T plasmid and then transferred it to pcDNA3 to evaluate its immunization with aluminum phosphate as an adjuvant in BALB/c mice. Hoseinian Khosroshahi et al. used this plasmid (pcDNA3-SAG1) and pcDNA3-ROP2 in a DNA vaccine cocktail approach to evaluate the immune response against toxoplasmosis (39). In another study, Chuang et al. investigated protective immunity of recombinant SAG1 protein into the plasmid pGEX-6P-1 and expressed as a GST fusion protein in BL21 (DE3) E. coli and then, rSAG1 was encapsulated into PLG (poly lactide-co-glycolide) microparticles using the double emulsion method and intraperitoneally injected into BALB/c mice (40). Qu et al. cloned the SAG1 gene into pcDNA3 and transformed into Salmonella typhimurium competent cells then assessed the immune responses of female ICR mice vaccinated by oral inoculation (32). Also, Meng et al. cloned the SAG1 gene and 14-3-3 gene of T. gondii into eukaryotic expression pBudCE4.1 then evaluated the protective immune responses of them in BALB/c mice (41).
pVAX1™ is a 3.0 kb plasmid vector that was constructed by modifying pcDNA™ 3.1 for use in the development of DNA vaccines. The vector was constructed to be consistent with the Food and Drug Administration (FDA) regulations. The vector was constructed to be consistent with the Food and Drug Administration (FDA) regulations. This vector allow high-copy number replication in E. coli and contains a human cytomegalovirus (CMV) immediate-early promoter for high-level expression in a wide range of mammalian cells, Bovine Growth Hormone (BGH), poly-adenylation signal for efficient transcription termination and poly-adenylation of mRNA and kanamycin resistance gene for selection in E. coli. Therefore, recently many investigators became interested to use the pVAX1 vector plasmid for DNA vaccine experimental designs. Hence, some antigens of T. gondii, including SAG1, ROP2, GRA4 and MIC3 have cloned into the pVAX1 plasmid and used for DNA vaccine (19).
Wu et al. constructed a multicomponent DNA vaccine by encoding GRA1 and SAG1 genes of T. gondii in the pVAX1 vector and evaluated the protective ability of this construction against toxoplasmosis in BALB/c mice, which were challenged with tachyzoites (42). Zhou et al. also cloned the SAG1 and GRA2 from T. gondii plus surface antigen of hepatitis B virus in the pVAX vector as a novel genetic adjuvant to evaluate immunization of those DNA plasmids against toxoplasmosis in BALB/c mice (43). In current study, the DNA sequence encoding fragment of T. gondii (RH) SAG1 was amplified by specific primer, including Kozak sequence and cloned into pVAX1 expression vector under a CMV promoter. The Kozak consensus sequence, GCCACCATGT, was introduced in 5′ of the SAG1 DNA, which was demonstrated useful earlier for translational control and optimization of expression vectors in an eukaryotic organism (44).
Sequence analysis of SAG1 cloned into the pVAX1 shows that the sequence has 100% identity with TgCatBr1strain, FOU strain, GUY-1992-RUB strain and high homology of 99% with RH strain. Then, the expression of this gene was confirmed by RT-PCR, indirect immunofluorescence test and western blot analysis. In this study, unlike other surveys, the aimed to express full length of SAG1 in the pVAX1 expression vector to evaluate its immunity with DNA vaccine in future investigations. These results confirmed that the recombinant plasmid was successfully constructed and expressed in vitro and recombinant TgSAG1 protein possessed immunological activity that can be used for DNA vaccine in further investigations.