1. Background
Escherichia coli is a part of the normal intestinal flora, but some E. coli types cause disease (1). Escherichia coli pathogenic strains are divided into two groups: intestinal pathogenic E. coli (DEC) and extra-intestinal pathogenic E. coli (ExPEC), causing urinary tract infections (UTI) (2). There are six DEC pathotypes including enterotoxigenic E. coli (ETEC), enteroaggregative E. coli (EAggEC), enteroinvasive E. coli (EIEC), enteropathogenic E. coli (EPEC), enterohemorrhagic E. coli (EHEC) and diffusely adherent E. coli (3). UTI is still the most common infection worldwide and causes diseases such as pyelonephritis and cystitis; however, many advances in diagnosis and prevention methods have been made (4). UTI occurs more commonly in women than men, and about 50% of women have at least one infection experience at some point in their lives. UTIs are caused mostly by UPEC (5).
It is estimated that the health care cost arising from bladder infections in young women is over one billion dollars in the United States (6). In addition, urinary tract infection also causes various complications during pregnancy for both the mother and the embryo (7-10). In fact, the most common cause of bacterial infections in children less than 90 days of age is UTI. The incidence of UTI in this age group has been estimated at around 1% in boys and 3% - 4% in girls (8, 11). Therefore, the most important precaution for reducing these problems is the rapid and accurate identification of infection for timely and correct medical interventions.
Diarrhea has remained a health care problem in developing countries, resulting in mortality, mostly in children younger than five years old (12). DEC is the major cause of diarrhea in developing countries, while it causes traveler’s diarrhea in developed countries (13, 14). It is estimated that there are about 2.5 million child deaths per year due to diarrhea (15, 16). In Iran, one of the major reasons for hospital admissions and deaths in children less than 5 years of age is diarrhea (17, 18). Based on phylogenetic analysis, the E. coli strain can be divided into four categories including A, B1, B2, and D (19, 20). DEC strains are derived from groups A, B1 and D, non-pathogenic commensal strains from A and B1, and extra-intestinal pathogenic strains usually belong to groups B2 and D (3, 19). Phylogenetic analysis for separation of pathogenic strains from non-pathogenic strains of E. coli is based on group-specific genes. Rapid and sensitive determination of phylogroups may be helpful in finding the infection origin, for epidemiological studies and to allow clinicians to predict disease progression and its complications (21).
Conventional assays, such as bacterial culture, have been the standard methods for detection of bacteria (22). Many upstream processes including enrichment, plating on selective agar and finally confirmational biochemical tests are needed when using these methods (23). The inexpensiveness and ability to detect only viable bacteria are the main advantages of these methods, but they are laborious, time consuming and not efficient, and researchers tend to use molecular methods such as mPCR (24). Rapidity, simplicity, sensitivity, specificity and simultaneous detection are the remarkable advantages of molecular methods (25-27). With respect to the importance of various health, social and economic aspects associated with DEC, there must be timely intervention and management of infection caused by DEC and UPEC. Thus, the need to develop rapid, sensitive and accurate detection methods, such as PCR, is very tangible.
2. Objectives
According to the previously mentioned issues, the aim of the current study was to apply a multiplex PCR assay so as to perform rapid and accurate molecular typing and phylogenetic typing of DEC and UPEC.
3. Materials and Methods
3.1. Sample Collection, Culture and DNA Extraction
A total of 200 stool samples from patients with diarrhea and 100 urine samples from patients with UTI, which were referred to Milad (Tehran, Iran) and Tohid (Sanandaj, Iran) hospitals, were cultured for 24 hours on MacConkey’s agar (Merck, Germany) bacterial growth medium at 37°C. Biochemical investigation, detected 175 cases as diarrhea and 50 cases as UTI. The current study was approved by the research committee of the school of allied medical sciences, Iran university of medical sciences (Tehran, Iran).
A single colony from each isolate or strain was inoculated into 7 - 10 mL of tryptic soy broth (Merck, Germany) and grown overnight at 28°C. DNA extraction using the boiling method was performed. In this method, 100 µL bacterial pellets were mixed with 400 µL distilled water and boiled at 90°C for 10 minutes. The mixture obtained from the previous stage was centrifuged at a speed of 5000 rpm for five minutes. The supernatants were used as a DNA template in multiplex PCR. 1% agarose gel electrophoresis and a spectrophotometer were used for observing and checking the quality of extracted DNAs.
3.2. PCR and Molecular Assay
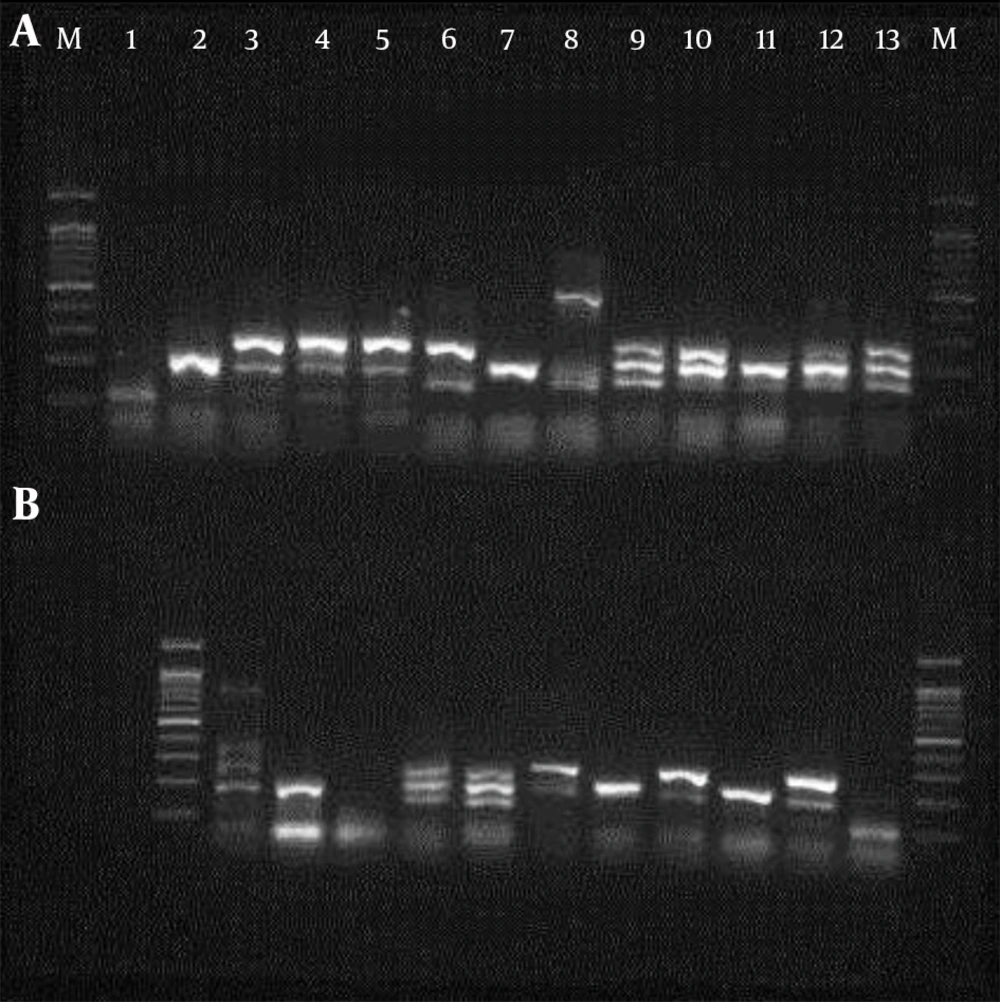
A molecular study using six pairs of primers (Takapouzist, Tehran, Iran) listed in Tables 1 and 2 was performed in two mPCR sets. Initially, a PCR with each primer as a confirmation test was made separately. Primers listed in Table 1 were used for determining of pathotypes and their frequency, and primers listed in Table 2 were used for phylogenetic study.
| Pathotype (Gene) | Primer Sequence (5′ → 3′) | Amplicon Size, bp |
|---|---|---|
| ETEC (ST) | 160 | |
| F: | TTT CCC CTC TTT TAG TCA GTC AAC TG | |
| R: | GGC AGG ATT ACA ACA AAG TTC ACA | |
| EAggEC (aggR) | 254 | |
| F: | GTA TAC ACA AAA GAA GGA AGC | |
| R: | ACA GAA TCG TCA GCA TCA GC | |
| ETEC (LT) | 330 | |
| F: | GGC GAC AGA TTA TAC CGT GC | |
| R: | CGG TCT CTA TAT TCC CTG TT | |
| EIEC (invE) | 382 | |
| F: | ATA TCT CTA TTT CCA ATC GCG T | |
| R: | GAT GGC GAG AAA TTA TAT CCC G | |
| DAEC (daaD) | 444 | |
| F: | TGAACGGGAGTATAAGGAAGATG | |
| R: | GTCCGCCATCACATCAAAA | |
| EPEC (eae) | 482 | |
| F: | TCA ATG CAG TTC CGT TAT CAG TT | |
| R: | GTA AAG TCC GTT ACC CCA ACC TG |
| Pathotype | Primer Sequence (5′ → 3′) | Anplicon Size, bp |
|---|---|---|
| ChuA | 279 | |
| F: | GACGAACCAACGGTCAGGAT | |
| R: | TGCCGCCAGTACCAAAGACA | |
| YjaA | 211 | |
| F: | TGAAGTGTCAGGAGACGCTG | |
| R: | ATGGAGAATGCGTTCCTCAAC | |
| TspE4C2 | 152 | |
| F: | GAGTAATGTCGGGGCATTCA | |
| R: | CGCGCCAACAAAGTATTACG |
The PCR procedure was performed as follows: each 25 µL of reaction mixture contained 3 µL of template DNA, 1 µL MgCl2 (Sinaclon, Iran), 0.17 µL of each primer, 1 unit of Taq DNA polymerase (Sinaclon, Iran), 0.5 µL dNTP Mix (Sinaclon, Iran), 2 µL 10× PCR buffer (Sinaclon, Iran) and 17 µL dd H2O. The reaction mixtures in the initial denaturation stage were heated at 96°C for five minutes and were amplified for 30 cycles using a gradient master cycler (Eppendorf, Hamburg, Germany) in stage 2. Each cycle was comprised of denaturation at 94°C for 30 seconds, annealing at 55°C (for aggR, daaD and invE genes) and 53°C (for ST, LT and eae genes) for 30 seconds, and extension at 72°C for one minute. The final extension was performed at 72°C for seven minutes. PCR products were analyzed by electrophoresis (100 V for 1 hour) on 1% gel agarose and stained with DNA green viewer (Aryatous, Iran).
The collected data were analyzed using SPSS version 16.0 for descriptive statistics (percentage, absolute and relative frequency, mean ± SD) and chi-square analyses.
4. Results
In the present study, a total of 200 isolated E. coli samples, including 100 from diarrhea and 100 E. coli samples from patients with UTI, were studied. The confirmation PCR results for each primer were identical to those achieved during the multiplex PCR procedure. The mPCR results obtained for different pathotypes of stool and urine samples are shown in Table 3 and Figure 1. The most frequent pathogenic gene in the UPEC samples was EAggEC (aggR), whose frequency was 23%. There was no eae (EPEC) gene found in the UPEC samples. In stool samples, there was no pathogenic gene detected in 9% of the samples, while 91% of the stool samples studied carried the pathogenic gene. ETEC was the most commonly isolated pathotype among fecal samples (ST, 62% and LT, 25%), while the least isolated pathotype (5%) was eae (EPEC). There was a significant difference between frequency of pathogenic genes in stool samples and urine samples, as shown by a chi-square test (P = 0.0005).
| Gene | Total of Samples | Stools Samples | Urine Samples |
|---|---|---|---|
| ETEC (ST) | 117 (67) | 109 (62) | 8 (8) |
| ETEC (LT) | 58 (33) | 44 (25) | 14 (14) |
| EAggEC (aggR) | 62 (35) | 39 (24) | 23 (23) |
| EIEC (invE) | 19 (11) | 13 (7) | 6 (6) |
| DAEC (daaD) | 27 (15) | 21 (13) | 6 (6) |
| EPEC (eae) | 10 (6) | 10 (5) | 0 |
aValues are expressed as frequency (%).
bThe chi-square statistic is 26.1497.
cThe result is significant at P < 0.01.
The frequency of simultaneously presented genes in the studied samples is shown in Table 4. In urine samples, eight samples carrying two pathogenic genes are presented simultaneously. In fecal samples, two samples carrying all four pathogenic genes, nine samples carrying three pathogenic genes and 35 samples carrying two pathogenic genes are presented simultaneously.
| Simultaneously Presented Genes | Urine Samples | Stool Samples | Total Samples |
|---|---|---|---|
| daaD, LT, aggR, ST | 0 | 2 | 2 |
| daaD, LT, ST | 0 | 2 | 2 |
| daaD, LT, aggR | 0 | 5 | 5 |
| invE, aggR, ST | 0 | 2 | 2 |
| LT, ST | 2 | 3 | 5 |
| LT, aggR | 3 | 4 | 7 |
| LT, daaD | 0 | 3 | 3 |
| ST, aggR | 1 | 4 | 5 |
| ST, eae | 0 | 2 | 2 |
| ST, daaD | 0 | 7 | 7 |
| aggR, eae | 0 | 2 | 2 |
| aggR, invE | 1 | 6 | 7 |
| aggR, daaD | 1 | 3 | 4 |
| daaD, eae | 0 | 1 | 1 |
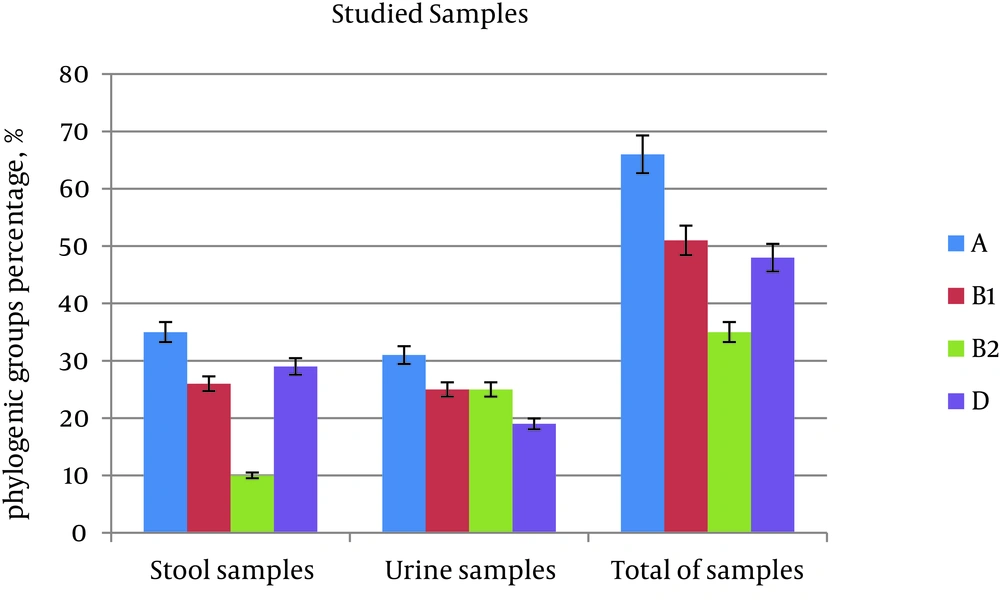
Results obtained from phylogenetic groups showed that group A was the most frequent in both urine samples (31%) and stool samples (35%). The frequency of group B1 in stool samples was 26%, while in urine it was 25%. The frequency of B1 in both samples is approximately the same and showed as the second most frequent group. The frequency of group B2 was 10% in stool samples and 25% in urine samples, while the frequency of group D in stool samples was 29%, and in urine sample it was 19%. Both of the groups (B2 and D) show the highest difference in frequency between stool and urine samples. There was a significant difference between the frequency of phylogenetic group distribution in stool and urine samples (P = 0.009) (Table 5 and Figure 2). The distribution of genes in phylogenetic groups was also different, so that ST had the highest frequency in group D. On the other hand, LT and aggR were the most frequent in group A, invE was the most frequent in group B1, and daaD was the most frequent in group B2 (Table 6).
| Phylogenetic Group | Total of Samples | Stool Samples | Urine Samples |
|---|---|---|---|
| A | 83 (33) | 51 (35) | 32 (31) |
| B1 | 63 (25) | 37 (26) | 26 (25) |
| B2 | 40 (16) | 14 (10) | 26 (25) |
| D | 62 (25) | 42 (29) | 20 (19) |
| Total | 248 (100) | 144 (100) | 104 (100) |
aValues are expressed as frequency (%).
bThe chi-square statistic is 11.5247.
cThe P value is 0.009202.
dThe result is significant at P < 0.01.
| Gene | Phylogenetic Groups | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | D | B2 | B1 | A | |||||||||||
| Positive | Negative | % | Positive | Negative | % | Positive | Negative | % | Positive | Negative | % | Positive | Negative | % | |
| ST | 57 | 46 | 55 | 13 | 13 | 50 | 10 | 7 | 59 | 14 | 10 | 58 | 20 | 16 | 56 |
| LT | 26 | 77 | 26 | 3 | 23 | 8 | 2 | 15 | 12 | 7 | 17 | 29 | 14 | 22 | 39 |
| aggR | 27 | 76 | 26 | 6 | 20 | 23 | 3 | 14 | 18 | 7 | 17 | 29 | 11 | 25 | 31 |
| invE | 13 | 90 | 13 | 4 | 22 | 15 | 3 | 14 | 18 | 5 | 19 | 21 | 1 | 35 | 3 |
| daaD | 11 | 92 | 11 | 0 | 26 | 0 | 3 | 14 | 18 | 3 | 21 | 13 | 5 | 31 | 14 |
| eae | 3 | 110 | 3 | 0 | 26 | 0 | 1 | 16 | 6 | 0 | 24 | 0 | 2 | 34 | 6 |
aThe chi square statistic is 13.188.
bThe P value is 0.355529.
cThe result is not significant (P < 0.05).
5. Discussion
Diarrhea and UTI are two major health problems caused by bacteria, and DEC and UPEC are the main players in development of these infections (11, 12). Phenotypic assays are normally used in most laboratories to characterize DEC and UPEC strains. However, these methods alone are not sufficient to identify all E. coli pathotypes (28). In addition, they are laborious and slow (26). Thanks to advances in technologies, a majority of microorganism genomes have been sequenced, and characterizations of these genomes provide researchers with accurate and rapid molecular methods for the identification of organisms based on detecting strain-specific genes among a large number of other organisms. Therefore, using molecular methods that are precise and sensitive, such as PCR, have recently gained attention for detection, speciating, typing, classifying or determining pathogenic E. coli. PCR has been applied and developed in several studies for categorization of pathogenic E. coli (28). In this study, multiplex PCR with two sets of primers was used; one set was comprised of primers for aggR, daaD and invE, and the other set consisted of primers for LT, ST and eae genes. It was found that these two primer sets were able to characterize the virulence factors of the gene and determine DEC pathotypes in 2 - 3 hours.
In many research studies, a different prevalence of DEC has been reported. In two research studies conducted on Bangladeshi and Jordanian children, the DEC prevalence was 40% and 34%, respectively (29, 30). In our study, the frequency of different pathotypes in urine and stool samples was significantly different (P < 0.01; Table 3). The results of the present study showed that ST (ETEC), with 62%, had a higher frequency in stool samples, and EAggEC (aggR) (23%) was the most frequent pathotype in UTI samples, while in urinary samples only two cases of ST were seen. Our results were consistent with some previous studies. A study conducted on 60 UTI samples in Iraq reported that 45 samples out of 60 were ETEC, and the LT gene was reported as the most frequent gene (31). Based on studies carried out in Brazil and several other countries, ETEC and EAEC were reported as the most common pathotypes in children with diarrhea, and EAEC was reported as a major cause of resistant acute diarrhea (32).
In another research study carried out on stool samples, the frequency of ETEC, EPEC and EIEC were reported as 16%, 8% and 1%, respectively (33). In several other studies, ETEC has been reported as the most common pathotype (34-36). In contrast, some studies have reported that EAEC is the most prevalent pathotype (37-41). Several studies carried out in 2001 and 2006 reported EPEC as the most common pathotype in patients with diarrhea, while ETEC was not reported in these studies (42-44). In numerous other studies, EPEC and EAEC were reported as the most common DEC pathotypes (45-47). The differences between our results and others may be attributed to pathogen strains, virulence factors, route of infection, difference in population selection and the sample size.
In this study, E. coli phylotypes were identified and isolated successfully, using multiplex PCR amplifying ChuA, YjaA and TspE4C2. This method helped us to identify the pathotype and can be used as a rapid and accurate method for the isolation of pathogenic strains of E. coli. Our phylogenetic results were the same as previous studies reporting Group B2 as a common phylogenetic group (48, 49). In a study conducted by Toval et al. (50), 55.8% of isolates belonged to phylogroups B2 and Hosseini et al. and Johnson et al. (51, 52) found that the frequency of phylogroups B2 and D was 71%. In our study, group B2 was the most prevalent, with 47.5%, and group B1, with only 2.5%, had the least prevalence.
Due to the high prevalence of diarrhea and UTI worldwide, and their importance in different aspects such as health, social and economic well-being, there exists a need to develop a rapid, sensitive and accurate diagnostic method for detection. Polymerase chain reaction (PCR) can improve the detection, and therefore, the management of these infections.