1. Background
Almost 10% of hospital-acquired infections are mainly caused by Pseudomonas aeruginosa (1). Acquired resistance is due to the production of plasmid-mediated Amp C β-lactamase, Metallo Β-Lactamase enzymes (MBL) and Extended Spectrum Β-Lactamase (ESBL) (2). Carbapenems are the elective drugs for treatment of multi-drug resistant (MDR) strains; recently, the increase of carbapenem-resistant P. aeruginosa has become a serious challenge worldwide (3). Furthermore, MBL are able to hydrolyze this category of antibiotics and their catalytic activities are not inhibited by inhibitors like sulbactam, clavulanic acid and tazobactam (4). However they are sensitive to metal chelates like EDTA, which are used to detect MBL activities of organisms (5). Since the late 1970s, Amp C β-lactamases have gained extended significance as one of the mechanisms of resistance in gram negative bacteria (6). Amp C enzymes are partially capable of hydrolyzing all β-lactams, poorly inhibited by clavulanic acid, and distinguished from ESBLs by their ability of cephamycins hydrolysis (7).
2. Objectives
Multidrug-resistant P. aeruginosa are the most prevalent bacterial isolates amongst burned and respiratory infected patients. Our study objective was to define the antibiotic susceptibility profiles of P. aeruginosa, as well as MBL and Amp C β-lactamases detection.
3. Methods
3.1. Bacterial Analysis
The study included one hundred and forty-seven (147) clinical specimens of isolates of P. aeruginosa collected between May 2013 and February 2014, from Suez Canal university hospital in Ismailia, Egypt, with different sources of infections. All P. aeruginosa samples were isolated by standard microbiological procedures, identified using API 20NE (BioMerieux, France), and stockpiled in Luria-Bertani broth medium (Merck, Germany) having 30% glycerol at -80°C.
3.2. Drug Susceptibility Testing
Drug susceptibility testing and interpretation were performed according to clinical laboratory standards institute guidelines (8), using disk diffusion method for antimicrobial agents, including Piperacillin (PRL), Ceftazidime (CAZ), Cefotaxime (CTX), Ceftriaxone (CRO), Cefepime (FEP), Gentamicin (CN), Amikacin (AK), Tobramycin (TOP), Polymyxin (PB), Norfloxacin (NOR), Aztreonam (ATM), Imipenem (IPM), Meropenem (MEM) and Piperacillin-Tazobactam (TZP) [Oxoid, England]. Multi-Drug Resistant P. aeruginosa isolates were resistant to at least three classes of the following compounds β-lactams, fluoroquinolones, and aminoglycosides. Pseudomonas aeruginosa ATCC 27853 was run simultaneously with the tested organisms for quality control of the susceptibility testing.
3.3. Phenotypic Detection of Metallo Β-Lactamase Production
Imipenem and meropenem resistant strains were screened for carbapenemase activity by Modified Hodge Test (MHT) (9). Positive P. aeruginosa strains were tested for MBLs production by Imipenem/EDTA double disk synergy test (10) and disk potentiation test (11).
3.3.1. Modified Hodge Test (MHT)
Suspension of overnight culture of E. coli ATCC 25922 was adjusted to 0.5 McFarland standard, using a sterile cotton swab on the surface of a Mueller-Hinton agar (Oxoid, England). After drying, 10 μg of imipenem disk was placed in the middle of the plate and the test organism was heavily streaked from center to periphery of the plate in four different directions, and it was allowed to stand for 15 minutes at room temperature. The plate was incubated overnight at 37°C. The presence of distorted zone of inhibition, a ‘cloverleaf shaped’ due to carbapenemase production by the test strain, was considered as positive results.
3.3.2. Imipenem-EDTA Double Disk Synergy Test (DDST)
The IMP-EDTA double disk synergy test was performed for detection of Metallo-β-lactamases. Liquid overnight culture of the tested isolate was adjusted to a turbidity of 0.5 McFarland standards, and spread on the surface of a MHA plate. After drying, a 10-μg imipenem disk, and a blank sterile filter paper disk (6 mm in diameter) were placed 10 mm apart from edge to edge. Ten microliters of 50 mM zinc sulfate solution was added to the 10-μg imipenem disk (MBLs requires divalent cations at the active site for their activation, usually zinc). Ten microliters of 0.5-M EDTA (Sigma, USA) solution was added to the blank filter paper disk. After overnight incubation at 37°C, the presence of a stretched growth inhibition zone between the two disks was interpreted as positive for MBL.
3.3.3. Disk Potentiation Test
Turbidity was adjusted to 0.5 McFarland standard of the tested strains and inoculated on Mueller Hinton agar plate. Two imipenem disks (10 μg) were placed on the plate wide apart, and 10 μL of 0.5-M EDTA solution was added to one imipenem disk. The inhibition zones of the imipenem and imipenem-EDTA disks were compared after 24 hours of incubation at 37°C. The increase in inhibition zone with the imipenem and EDTA disk was ≥ 7 mm when compared to the imipenem disk alone; it was deliberated as MBL-positive isolates.
3.4. Detection of AmpC β-lactamase
Metallo β-lactamase producing isolates were screened for Amp C β-lactamase; cefoxitin (Oxoid, England) inhibition zone diameter < 18 mm were considered as positive for Amp C β-lactamase production (12).
3.4.1. Amp C Test
Test principle was established on use of Tris-EDTA to permeabilize a bacterial cell and release β-lactamases into the outside environment. Amp C (13) disks (disk of filter paper 6-mm in diameter containing Tris-EDTA) were prepared by applying 20 μL of a 1:1 mixture of saline and 100 μL Tris-EDTA to sterile filter paper disks, permitting the disks to dry, and storing them at 8°C (14).
An adjusted 0.5-McFarland suspension standard of overnight culture of cefoxitin-susceptible E. coli ATCC 25922 was made and a lawn of culture was inoculated on the surface of a Mueller-Hinton agar plate (8). Amp C disks were rehydrated with 20 μL of saline, and several colonies of P. aeruginosa were applied to a disk. The cefoxitin disk (30 μg) was placed on the inoculated surface of the MHA. The inoculated Amp C disk was nearly touching the cefoxitin antibiotic disk. The plate was incubated overnight at 37°C.
3.4.2. Disk Antagonism Test
Inducible Amp C β-lactamases was detected as, 0.5 McFarland of test (15) isolate was swabbed on MHA plate, ceftazidime (30 μg), and cefoxitin (30 μg) disks were placed 20 mm apart from center to center. Presence of inhibition zone blunting in the ceftazidime disk was considered inducible Amp C β-lactamase.
3.4.3. Amp C Inhibitor Method (12)
A disk containing 30 μg of cefoxitin and another containing cefoxitin with 3-Aminophenylboronic Acid (APB) (16), were placed on the agar. Inoculated plates were incubated overnight at 35°C. Comparison of zone size of cefoxitin - APB disk and cefoxitin only disk was more ≥ 5 mm recorded as Amp C β-lactamase producer.
3.5. Minimum Inhibitory Concentrations Determination of Carbapenem
Carbapenems MICs, determined for MBLs producers by the agar dilution method, were graded serially to obtain drug concentrations ranging from 1024 to 0.125 μg/mL of the respective commercial preparation of imipenem [500 mg powder, Manufacturers: Glaxo Smithklein, Cairo, Egypt] and meropenem [500 mg powder, Astra Zeneca pharma, Cairo, Egypt], and were taken for the study of antibiotics, according to the Clinical and Laboratory Standards Institute (CLSI) (8).
4. Results
One hundred and forty-seven (147) non-duplicate P. aeruginosa clinical isolates were collected from Suez Canal university hospital. The clinical specimens were collected from clinically diagnosed patients and separated into six groups, according to the source of infection as shown in Table 1.
| Isolated Group | Sources of P. aeruginosa Isolates | Number of Isolates (n = 147) | Percentage |
|---|---|---|---|
| Group I | Wounds & pus swabs | 63 | 43% |
| Group II | Sputum | 34 | 23% |
| Group III | Urine | 29 | 20% |
| Group IV | Blood sample | 11 | 7% |
| Group V | Ear exudate | 7 | 5% |
| Group VI | Vaginal discharge | 3 | 2% |
| Antimicrobial Agent(s) | Concentration (µg) | Resistant, No. (%) | Intermediate, No. (%) | Sensitive, No. (%) |
|---|---|---|---|---|
| PRL | 100 | 83 (56) | - | 64 (43.5) |
| CAZ | 30 | 111 (75.5) | 16 (11) | 20 (14) |
| CTX | 30 | 141 (96) | 4 (3) | 2 (1.3) |
| CRO | 30 | 121 (82) | 11 (7) | 15 (10) |
| FEP | 30 | 97 (66) | 8 (5) | 42 (28.5) |
| CN | 10 | 27 (18) | 5 (3.4) | 115 (78) |
| AK | 30 | 28 (19) | 6 (4) | 113 (77) |
| TOP | 10 | 29 (20) | 8 (5) | 110 (75) |
| PB | 300 IU | 2 (1.3) | - | 145 (99) |
| NOR | 10 | 16 (11) | 2 (1.3) | 129 (88) |
| ATM | 30 | 23 (16) | 27 (18) | 97 (66) |
| IMP | 10 | 11 (7) | 2 (1.3) | 134 (91) |
| MEM | 10 | 35 (24) | 9 (6) | 103 (70) |
| TZP | 100/10 | 26 (18) | - | 121 (82) |
Abbreviations: PRL, Piperacillin; CAZ, Ceftazidime; CTX, Cefotaxime; CRO, Ceftriaxone; FEP, Cefepime; CN, Gentamicin; AK, Amikacin; TOP, Tobramycin; PB, Polymyxin; NOR, Norfloxacin; ATM, Aztreonam; IPM, Imipenem; MEM, Meropenem; TZP, Piperacillin Tazobactam.
4.1. Metallo-β- Lactamase-producing Pseudomonas aeruginosa Isolates
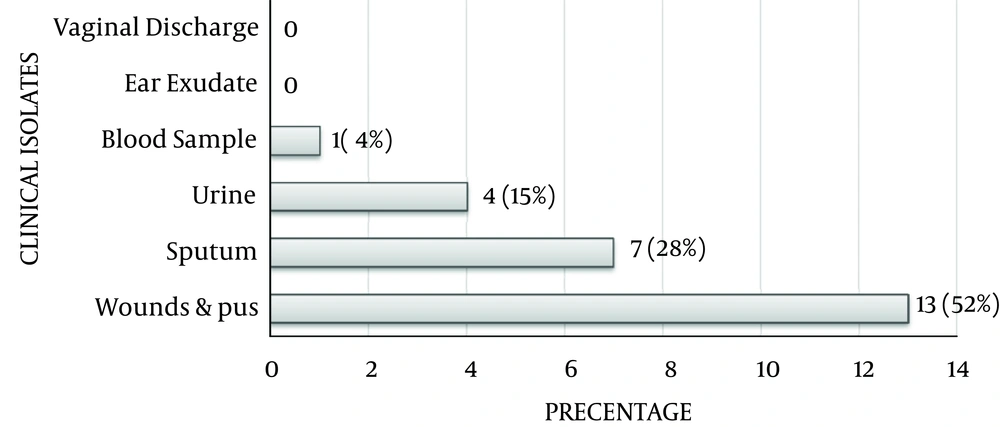
Thirty-nine (39, 26.5%) out of 147 were carbapenem (IMP & MEM) resistant P. aeruginosa isolates. Metallo-β- Lactamases producers were 25 isolates (17%), and in relation to clinical specimens shown in Figure 1, which were confirmed by imipenem-EDTA double disk synergy test and disk potentiation test.
4.2. Carbapenem Minimum Inhibitory Concentrations
Minimum Inhibitory Concentration determination for imipenem and meropenem was done by the agar dilution technique; (39) Carbapenemase-producing P. aeruginosa isolates are summarized in Table 3.
| Antibiotics | Minimum Inhibitory Concentration in μg/mL | ||||||
|---|---|---|---|---|---|---|---|
| ≤ 2 | 4 | 8 | 16 | 32 | 64 | ≥ 128 | |
| Imipenem | 9 | 4 | 7 | 7 | 3 | 6 | 3 |
| Meropenem | 2 | 8 | 7 | 8 | 8 | 2 | 4 |
4.3. Metallo-β-Lactamases Produced by Pseudomonas aeruginosa in Relation to Age
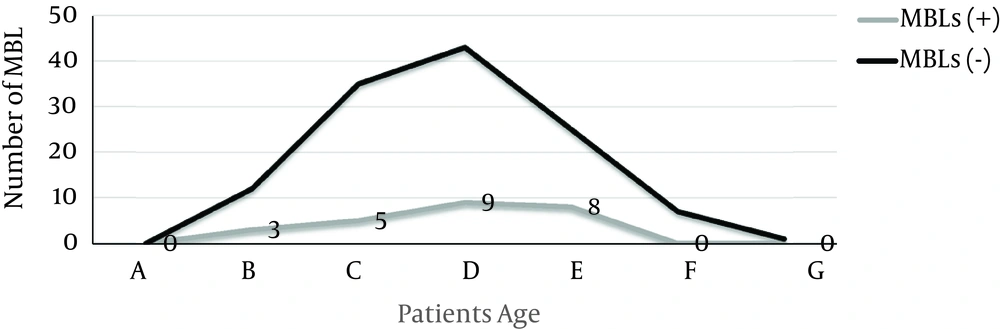
Patients infected with (MBL) P. aeruginosa (68%, 17/25 patients) mainly belonged to the 51 to 70 year-old age group, as detailed in patients age distribution curve of Figure 2. In addition, MBLs prevalence in males was 60% (15/25) and in females was 40% (10/25).
4.4. AmpC β -lactamase Detection
Metallo β-lactamase positive isolates (17) were screened for co-existence of Amp C. The potential Amp C β-lactamase producers, detected by the cefoxitin-screening test, were seven (28%) positive isolates. Among the seven screening positive isolates, one (4%) P. aeruginosa isolate revealed the presence of inducible Amp C β-lactamases by disk antagonism test, and plasmid mediated Amp C was detected in five (20%) P. aeruginosa isolates.
5. Discussion
Pseudomonas aeruginosa infection is a major cause of serious complications in hospitalized patients of developing countries (18, 19). Metallo-β-Lactamases have been identified from clinical isolates worldwide. Senda et al. reported an increasing frequency over the earlier few years, and bacteria producing these enzymes have been responsible for persistent nosocomial outbreaks that were accompanied by severe infections (20). In our study, the commonest specimen was wound, while pus swabs had a prevalence of 43% (63/147 isolates) and sputum swab 23% (34/147 isolates), followed by other specimens. These findings are consistent with other studies where P. aeruginosa was found frequently to cause suppurated skin and respiratory infections (21, 22).
Our results report that 26.5% (39/147) of P. aeruginosa strains were resistant to carbapenem antibiotics (imipenem & meropenem) of which, 64% (25/39) were detected as MBL-producers, which is much higher than studies conducted by Navneeth et al., (23), and Hodiwala et al., (24), who revealed 12% and 21% MBL-mediated imipenem resistance in P. aeruginosa. In our study the resistance rates of cefotaxime, ceftazidime, cefepime, piperacillin, aztreonam and meropenem were 98.6%, 86%, 71.4%, 56%, 34% and 30%, respectively. Behera et al. reported 70% resistance to ceftazidime, 75% to piperacillin, 59% to piperacillin/tazobactam, 74% to amikacin, 81% to cefepime, and 69% to aztreonam (25).
The sensitivity testing toward polymyxin, imipenem, norfloxacin, piperacillin-tazobactam, and gentamicin were 99%, 91%, 88%, 82%, and 78%, respectively. In a previous study by Dardi and Wankhede, higher sensitivity rate was reported towards amikacin (83.3%), meropenem (81.7%), tobramycin (80%) and cefepime (66.7%) (26). Multi-Drug Resistance in our study was 64% (16/25), nearly similar to the study of Anvarinejad et al., which reported MDR of 63.5% (17). In the present study, the most common age group affected by MBLs was > 51 year-olds with a prevalence of 68% (17/25), and males with prevalence of 60% (15/25) were more frequently affected than females with prevalence of 40% (10/25), with, male: female ratio being 3: 2. Niranjan et al., showed that MBLs were more prevalent in the age group of 10 to 11 year-olds, with prevalence of 29% (10/34) (27).
Males were 64.7% (22/34) while females were 35.3% (12/34) with male: female ratio being 1.8: 1. Deba et al. in their study on MBLs detection reported that male: female ratio was 1.2: 1 and the most common age group was > 60 year-olds (46.6%) (28). Prevalence of Amp C β-lactamases among MBLs-producing P. aeruginosa isolates was 28% (7/25), which was lower than the study conducted by Noyal et al., that reported 46.9% (15/32) were Amp C β-lactamase and MBLs producers (29). Therefore, Amp C β-lactamase could be a significant causative factor for carbapenemase resistance between the isolates in our hospital similar to other studies (30, 31).