1. Background
Public health is globally in danger due to re-emergence of infectious diseases and also because of the antibiotic resistance among microbes (1). Enterococci are less virulent as compared to other pathogens, but they are fourth in causing nosocomial infections from the 1990s. Extensive use of invasive devices in patients and the irrational use of broad-spectrum antimicrobials have brought enterococci among important drug-resistant nosocomial pathogens. Therefore, enterococci can survive and reproduce freely in hospital and health care settings (2).
Two enterococcal species i.e. Enterococcus faecalis and E. faecium are frequent commensals in the gastrointestinal tract (GIT) of humans. Normally 90% - 95% E. faecalis are frequently isolated from faeces of normal healthy individuals, whereas E. faecium are isolated less frequently (5% - 10%) (3). These enterococci have been recognised as one of the important causes of endocarditis accounting for 5% - 20% of cases (4). During the past decade, there is an increase in occurrence of enterococci in hospital settings. In USA, enterococci were reported to be second in causing nosocomial infections and urinary tract infections (UTIs). It is also the second most frequent cause of intra-abdominal and intra-pelvic abscesses or post-surgery wound infections (5).
Enterococci are well-known for resistance to aminoglycosides, cephalosporins, clindamycin, erythromycin, tetracycline and are partially susceptible to glycopeptides, penicillin and ampicillin (6). Extensive resistance has been developed among the isolates of E. faecalis and E. faecium (isolated from humans, poultry and dogs) to tetracycline, chloramphenicol, kanamycin, macrolides and streptomycin (7). This is because of the exogenic genes and mutations that resulted in resistance (8).
Major problems have been aggravated by their amassed resistance to glycopeptides (teicoplanin and tancomycin) (9) and the emergence of vancomycin-resistant enterococci (VRE) as nosocomial pathogens are associated with high mortality rates across the globe (10). Five different kinds of vancomycin resistance genes have been found in enterococci (VanA, VanB, VanC, VanD and VanE) (11).
With the limitations in antibiotic therapy due to increase in resistance in such microbes, focus is now on the alternative treatment regime. Currently inorganic metal oxides like calcium oxide (CaO), magnesium oxide (MgO), titanium oxide (TiO2) and zinc oxide (ZnO) have fascinated attentions as antimicrobial, anticancer and antiprotozoal agents due to their stability and safety (12-14). Many of these are used in the formulation of personal-care products (15-17). However, there are only a few studies which shows the use of combination of metal nanoparticles and particles especially ZnO with antibiotics against bacteria (18). Moreover, many studies are available on using silver nanoparticles combined with different antibiotics against both Gram positive and Gram negative bacteria (19-23). Thus, the present study was designed to evaluate the anti-enterococcal activity of nanoparticles alone and in combination with antibiotics towards the development of new biocidal combination against pathogens.
2. Objectives
Present study deals with the efficacy of antibiotic-nanoparticle combination against the clinical isolates of vancomycin resistant enterococci (VRE). The current study effectively evaluated the anti-enterococcal activity of metallic nanoparticles and their combination with antibiotics with the aim to search for new biocidal combinations.
3. Materials and Methods
3.1. Specimen Collection
Enterococcal clinical isolates were obtained from a previous study (2). The samples were collected from the anterior nares and perirectal area of the patients of ICU. Three ATCC strains were used as a control for antibiotic and nanoparticles susceptibility testing; ATCC 51229 VRE-E. faecalis (vanB), ATCC 29212 vancomycin susceptible enterococci (VSE)-E. faecalis and ATCC 51559 VRE-E. faecium (vanA).
3.2. Biochemical Identification
The samples were inoculated onto brain heart infusion agar (BHI) (Oxoid, UK) and bile esculin agar (BEA) (Oxoid, UK). The plates were incubated aerobically at 45°C for 18 - 24 hours. Pin point colonies with black zone around BEA were identified as enterococci by negative catalase and coagulase tests and growth on Mueller Hinton agar (MHA) (Oxoid, UK) with 6% NaCl at 45°C. The confirmation of presumptive positive enterococci was done using VITEK by recommended procedure (VITEK 2 System version 5.01, BioMerieux).
3.3. Identification by PCR
The identification of E. faecalis and E. faecium was done using PCR by amplifying D-Ala:D-Ala ligase gene (ddlE. faecalis and ddlE. faecium) and VRE was identified by targeting vancomycin-resistant gene (vanA and vanB). Primers were purchased from Sigma Genosys (Sigma Aldrich, USA) adapted from a previous study by Kariyama et al. (2000) (24). DNA was extracted with the help of Wizard® Genomic DNA purification kit (Promega Corporation, USA) according to manufacturer’s instructions. The D-Ala:D-Ala ligase gene was amplified with primers ddlE. faecalis(F)= ATCAAGTACAGTTAGTCT, and ddlE. faecalis(R) = ACGATTCAAAGCTAACTG at 941 bp for E. faecalis whereas the primer ddlE. faecium (F) = TTGAGGCAGACCAGATTGACG and ddlE. faecium (R) = TATGACAGCGACTCCGATTCC gave amplicon at 658 bp for E. faecium. VanA genes were amplified with primer vanA-F = GGGAAAACGACAATTGC, and vanA-R = GTACAATGCGGCCGTTA while vanB genes were carried out with primer vanB-F = GTGCTGCGAGATACCACAGA, and vanB-R = CGAACACCATGCAACATTTC with an amplification of 732 bp and 635 bp respectively.
Biometra T1 Thermocycler (Biometra, Germany) was used for amplification. The cycling conditions were as follows: cycle 1: 95°C for 4 minutes cycle 2 - 30: denaturation, 95°C, 30 seconds, annealing 52°C, 60 seconds, extension, 72°C, 2 minutes with the final extension of 7 minutes. Afterwards, the PCR product was run on 1% agarose gel. DNA ladders (O’Gene Ruler, Thermo Fisher Scientific, UK) of 100 bp and 1 kb were used to compare the amplified fragments. The gel was run for electrophoresis at 100 V for 30 minutes and viewed under Molecular Imager Gel Doc XR+ System, Bio-Rad Laboratories, US.
3.4. Antimicrobial Susceptibility Testing
Kirby-Bauer modified Disc diffusion method was used to perform antimicrobial susceptibility testing for all the isolates according to CLSI 2009 recommended direct colony suspension method for disc diffusion. The antibiotic discs used were azithromycin (AZM15), ciprofloxacin (CIP5), erythromycin (E15), imipenem (IPM10), linezolid (LZD30), penicillin G (P10), quinupristin/dalfopristin (QD15), teicoplanin (TEC30), tetracycline (T30) and vancomycin (V30). Mueller Hinton broth (MHB) was used for both antibiotic susceptibility and nanoparticles antimicrobial assay. Broth dilution method was used for MIC using 96-well plate reader Synergy 2 (BioTek, USA) with Gen5 data analysis software. Antibiotics used for MIC were ciprofloxacin, erythromycin, methicillin and vancomycin (Sigma Aldrich, USA). Inoculum used was at a concentration of 106 cfu/mL per well. Following inoculation in 96-well plates under continuous shaking, the optical density (OD) of inoculated isolates was monitored at 600 nm after every 15 minutes with a final reading after 24 hours.
3.5. Antibacterial Activity of Nanoparticles
CaO (< 160 nm and < 50 nm), MgO (< 160 nm and < 50 nm) and ZnO particles (< 50 nm, 100 nm and 5 μm) were purchased from Sigma Aldrich, US. The growth curves of bacterial cells exposed to nanoparticles were examined to evaluate the antibacterial activity of these nanoparticles. On MHB growth medium, different concentrations of particles were used i.e. 0.3125 to 30 mM for CaO and MgO nanoparticles, while 0.3125 to 7 mM for ZnO particles. The concentration of bacterial cells was adjusted to 106 cfu/mL and incubated in a shaking incubator and read on 96-well plate reader Synergy 2 at 37°C for 24 hours. Growth curves were attained through serial monitoring of the OD at 600 nm. Nanoparticle-free media with bacterial cultures under the same growth conditions were used as control.
3.6. Antibacterial Activity of Combination of Antibiotics With Nanoparticles
Nanoparticles concentrations were selected depending upon the MICs of that nanoparticle for respective strain and were mixed with serially diluted antibiotics in the wells of a 96-well plate. Concentrations of ZnO particles used were 0.312 to 1.25 mM and 10 mM for CaO and MgO nanoparticles. Cultures were incubated on shaker incubator at 37°C with medium speed. ODs of the cultures were measured every 15 minutes using 96-well plate reader at 600 nm with a final kinetic reading at 24 hours.
4. Results
Out of total 12 clinical isolates of enterococci, 3 were E. faecalis and 9 E. faecium including 4/12 VRE and 8/12 vancomycin sensitive enterococci (VSE). D-Ala:D-Ala ligase gene amplification was successfully done with ddlE. faecalisprimers at 941 bp and ddlE. faeciumat 658 bp. Vancomycin resistance gene vanA was amplified in both E. faecalis and E. faecium at 732 bp. The vanB primer gave no amplification in both strains of enterococci except the control strain.
4.1. Antibiotic Resistance Pattern
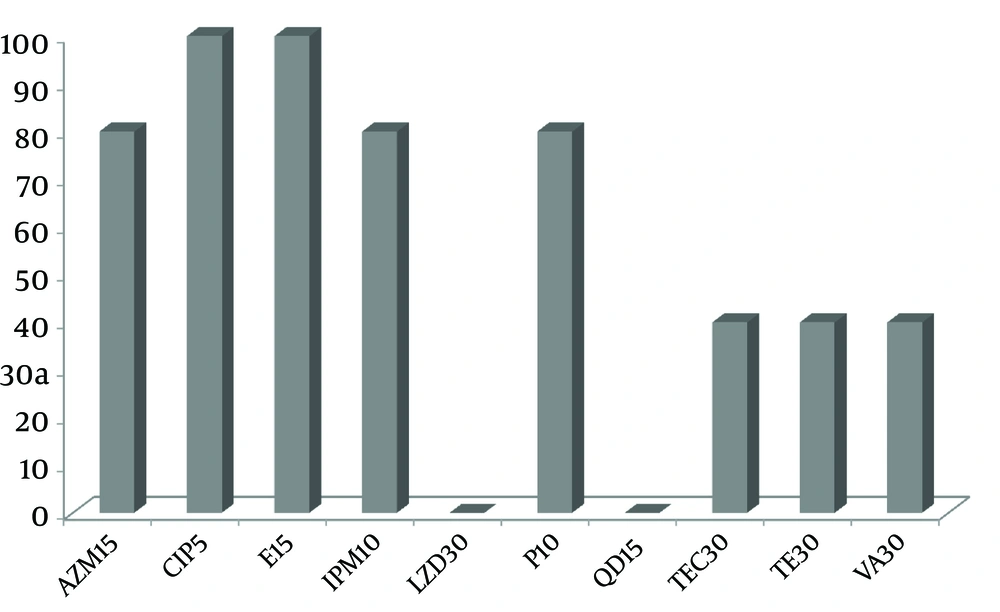
All of the enterococci were 80 to 100% resistant to azithromycin, ciprofloxacin, erythromycin, imipenem and penicillin G while, 40% resistance was found against tetracycline, teicoplanin and vancomycin. None of the isolates were resistant to linezolid and quinupristin/dalfopristin (Figure 1).
4.2. Minimum Inhibitory Concentrations of Antibiotics
The MIC for ciprofloxacin ranged from 16 to 256 μg/mL indicating resistance (≥ 4 μg/mL) of the isolates to ciprofloxacin. MICs of erythromycin ranged from 1024 to 2048 μg/mL (≥ 8 μg/mL). While all the isolates were resistant to methicillin (≥ 8 μg/mL) with an MIC of 32 to 256 μg/mL. Among E. faecalis and E. faecium, 6/15 were VRE (≥ 8 μg/mL), including two reference strains. While 9/15 were VSE with MIC ≤ 8 μg/mL. MICs of vancomycin varied significantly among the isolates (Table 1).
Abbreviations: AD, Antibiotic dilutions; Cip, Ciprofloxacin; E, Erythromycin, Met, Methicillin; Van, Vancomycin.
aValues are presented as No. (%). No., Number of isolates; and %, percentage of resistant isolates in total isolates (N = 15).
bBreakpoint concentration.
4.3. Minimum Inhibitory Concentrations of Nanoparticles
MICs of ZnO against the enterococci ranged from 5 to 7 mM for < 5 μm and < 100 nm sized ZnO particles while 2.5 to 5 mM for < 50 nm sized nanoparticles. With < 50 nm ZnO, 10/15 isolates were inhibited at 2.5 mM while remaining at 5 mM. The MICs of CaO and MgO nanoparticles were higher as compared to ZnO particles and were in the range of 15 to 25 mM. CaO nanoparticles inhibited 10/15 isolates at 15 mM and remaining at 20 mM. MgO nanoparticles inhibited 8/15 of isolates at 20 mM, 2/15 and 5/15 at 15 mM and 25 mM respectively (Table 2).
| Concentration, mM | CaO, < 160 nm | MgO, < 50 nm | ZnO, < 50 nm | ZnO, < 100 nm | ZnO, < 5 μm |
|---|---|---|---|---|---|
| 2.5 | - | - | 10 (66.7) | - | - |
| 5 | - | - | 5 (33.3) | 9 (60) | 8 (53.3) |
| 6 | - | - | - | 5 (33.3) | 3 (20) |
| 7 | - | - | - | 1 (6.7) | 4 (26.7) |
| 15 | 10 (66.7) | 2 (13.3) | - | - | - |
| 20 | 5 (33.3) | 8 (53.3) | - | - | - |
| 25 | - | 5 (33.3) | - | - | - |
aValues are presented as No. (%). No., Number of isolates; and %, percentage of resistant isolates in total isolates.
4.4. Antibacterial Activity of Combination of Antibiotics With Nanoparticles
Different combinations of nanoparticles with different concentrations of ciprofloxacin, erythromycin, methicillin and vancomycin were tested against enterococcal isolates. Data of all the concentrations of nanoparticles applied on strains are not shown, except that of 0.625 mM, 1 mM, 1.25 mM, 1.5 mM, 2 mM, and 2.5 mM which efficiently reduced the MICs of the antibiotics. ZnO nanoparticles at concentrations of 1 mM (< 50 nm), 1.5 mM (< 100 nm) and 2.5 mM (< 5 μm) were found to be inhibitory with different concentrations of ciprofloxacin. These concentrations of ZnO effectively reduced the MICs range of ciprofloxacin from 16 - 256 μg/mL to 2 - 16 μg/mL, 4 - 32 μg/mL and 4 - 256 μg/mL for all three sized particles respectively. Similarly, 4 - 256 μg/mL MICs range was observed when ciprofloxacin was combined with 10 mM of CaO and MgO (Tables 3 and 4).
| AD, μg/mL | CIP, mM | E, mM | Met, mM | Van, mM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1, (ZnO < 50 nm) | 1.5, (ZnO < 100 nm) | 2.5, (ZnO < 5 μm) | 1, (ZnO < 50 nm) | 1.25, (ZnO < 100 nm) | 2.5, (ZnO < 5 μm) | 1.25, (ZnO < 50 nm) | 2, (ZnO < 100 nm) | 2.5, (ZnO < 5 μm) | 0.625, (ZnO < 50 nm) | 01, (ZnO < 100 nm) | 1.25, (ZnO < 5 μm) | |
| 0.5 | - | - | - | - | - | - | - | - | - | 5 (33.3) | 2 (13.3) | 2 (13.3) |
| 1 | - | - | - | - | - | - | - | - | - | 5 (33.3) | 3 (20) | 3 (20) |
| 2 | 3 (20) | - | - | - | - | - | - | - | - | - | 5 (33.3) | 4 (26.7) |
| 4 | 4 (26.7) | 3 (20) | 2 (13.3) | - | - | - | - | - | - | - | - | 1 (6.7) |
| 8 | 3 (20) | 4 (26.7) | 5 (33.3) | - | - | - | 4 (26.7) | 2 (13.3) | 2 (13.3) | - | - | - |
| 16 | 5 (33.3) | 3 (20) | 3 (20) | - | - | - | 2 (13.3) | - | 1 (6.7) | - | - | |
| 32 | - | 5 (33.3) | 3 (20) | - | - | - | - | - | 2 (13.3) | 4 (26.7) | 1 (6.7) | 1 (6.7) |
| 64 | - | - | - | - | - | - | 11 (73.3) | - | - | - | 4 (26.7) | 4 (26.7) |
| 128 | - | - | - | 5 (33.3) | 9 (60) | 10 (66.7) | - | 11 (73.3) | 11 (73.3) | - | - | - |
| 256 | - | - | 2 (13.3) | - | 1 (6.7) | - | - | - | - | - | - | - |
| 512 | - | - | - | 10 (66.7) | 5 (33.3) | 5 (33.3) | - | - | - | - | - | - |
Abbreviations: AD, Antibiotic Dilution; Cip, Ciprofloxacin; E, Erythromycin, Met, Methicillin; Van, Vancomycin.
aValues are presented as No. (%). No., Number of isolates; and %, percentage of resistant isolates in total isolates.
| AD, μg/mL | CIP | E | Met | Van | ||||
|---|---|---|---|---|---|---|---|---|
| CaO, < 160 nm | MgO, < 50 nm | CaO, < 160 nm | MgO, < 50 nm | CaO, < 160 nm | MgO, < 50 nm | CaO, < 160 nm | MgO, < 50 nm | |
| 0.5 | - | - | - | - | - | - | 2 (13.3) | 2 (13.3) |
| 1 | - | - | - | - | - | - | 3 (20) | 3 (20) |
| 2 | - | - | - | - | - | - | 4 (26.7) | 4 (26.7) |
| 4 | 1 (6.7) | 1 (6.7) | - | - | - | - | 1 (6.7) | 1 (6.7) |
| 8 | 1 (6.7) | 6 (40) | - | - | - | - | - | - |
| 16 | 6 (40) | 1 (6.7) | - | - | 2 (13.3) | 2 (13.3) | - | - |
| 32 | -- | - | - | - | 2 (13.3) | 2 (13.3) | 1 (6.7) | 1 (6.7) |
| 64 | - | - | - | - | - | - | 4 (26.7) | 4 (26.7) |
| 128 | - | - | 5 (33.3) | 8 (53.3) | 11 (73.3) | 11 (73.3) | - | - |
| 256 | 7 (46.7) | 7 (46.7) | - | - | - | - | - | - |
| 512 | - | - | 10 (60.7) | 7 (46.7) | - | - | - | - |
Abbreviations: AD, Antibiotic Dilution; Cip, Ciprofloxacin; E, Erythromycin, Met, Methicillin; Van, Vancomycin.
aValues are presented as No. (%). No., Number of isolates; and %, percentage of resistant isolates in total isolates.
With erythromycin, ZnO nanoparticles were found inhibitory at concentrations of 1 mM, 1.25 mM and 2.5 mM respectively by < 50 nm, < 100 nm and < 5 μm. There was an effective reduction in the MICs of erythromycin from 1024 - 2048 μg/mL to 128 - 512 μg/mL and same improvement with that of 10 mM of CaO and MgO.
Methicillin in combination with ZnO particles at concentrations of 1.25 mM (< 50 nm), 2 mM (< 100 nm) and 2.5 mM (< 5 μm) effectively reduced the MICs from 32 - 256 μg/mL to 8 - 64 μg/mL by < 50 nm and < 100 nm and 8 - 128 μg/mL by < 5 μm size particles. With 10 mM of CaO and MgO, the MICs were reduced to 16 - 128 μg/mL.
Vancomycin was also combined with ZnO particles in different concentrations. Most effective concentrations were 0.625 mM (< 50nm), 1 mM (< 100 nm) and 1.25 mM (< 5 μm). MICs of vancomycin against VRE were reduced from 256 - 512 μg/mL to 16 - 32 μg/mL with < 50 nm ZnO and to 32 - 64 μg/mL by < 100 nm and < 5 μm ZnO particles. Upon VSE, MICs were reduced from 2 - 4 μg/mL to 0.5 - 4 μg/mL with all these particles. Both CaO and MgO at 10 mM concentrations were found effective in reducing the MICs of VRE to 32 - 64 μg/mL range while for VSE it was the same as for ZnO nanoparticles (Tables 3 and 4).
5. Discussion
Multidrug resistant enterococcal isolates from hospitalized patients were collected, identified and used to study the anti-enterocoocal activity of metallic particles alone and in combination with antibiotics.
By disc diffusion, high resistance was found in both E. faecalis and E. faecium isolates against different antibiotics that was in accordance with the previous study of Karmarkar et al. (2004) (25). All the isolates were susceptible to linezolid and quinupristin/dalfopristin while resistant against ciprofloxacin and methicillin with MICs. A previous study reported 46.9% enterococci having MIC of 16 - 128 mg/L against ciprofloxacin, which is comparatively a bit less than the present study (26). With methicillin, enterococci are intrinsically resistant as mentioned in a previous study of Jones et al. (2008) (27) thus, all of the strains showed high MICs range.
In the current study, MICs of erythromycin was comparatively higher than previously identified enterococci (28). There were six enterococcal isolates (VRE) having MIC more than > 8 μg/mL (breakpoint concentration) with vancomycin and having similar MICs (32 to 512 mg/L) reported in another study of Aleyasin et al. (2007) (29).
Present study supports previous findings that CaO, MgO and ZnO particles have tremendous bactericidal properties (30-32). The results showed that the sizes and concentrations of CaO, MgO and ZnO particles have a significant role in antibacterial activity. Enterococci were effectively inhibited by ZnO nanoparticles. A study by Makhluf et al. (2005) reports the inhibition S. aureus and E. coli by MgO nanoparticles (25 nm) at a concentration of 1 mg/mL (24.8 mM) while in the present study, low concentrations and large size nanoparticles have successfully inhibited enterococci (31). Similar effects were observed in case of CaO nanoparticles. In another study by Padmavathy and Vijayaraghavan (2008), it was found that the microbial inhibition increased significantly with smaller size ZnO nanoparticles (12 nm) at 1 mM concentration against E. coli (33). In contrast, the present study evaluated that higher concentrations and large sized (≤ 50 nm) nanoparticles efficiently inhibited the Gram-positive organism. This may also be due to the difference in Gram-reactions, which needs further evaluation.
Metal-based drug development is a promising pharmacological application (34). So by taking this aspect into consideration, the current study employed the physical combination of CaO, MgO and ZnO particles with different antibiotics. Many studies have shown that metal nanoparticles combined with antibiotics have better effects against both Gram positive and Gram negative bacteria especially that of silver nanoparticles combined with antibiotics (streptomycin, ampicillin, amoxicillin, ciprofloxacin, imipenem, gentamycin, vancomycin, trimethoprim, erythromycin, and tetracycline) (20-22, 35, 36). But, literature is limited in representing data about other metals especially that of zinc in combination with different antibiotics. In the current study, the combination of antibiotic-nanoparticles showed greater microbial inhibition than the particles and antibiotics alone.
The most effective one was found to be ZnO < 50 nm nanoparticles. Previously, a study evaluated synergistic antimicrobial effects of ZnO nanoparticles with different antibiotics against S. aureus and E. coli using disk diffusion method which showed that ZnO nanoparticles (20 - 45 nm) at a concentration of 500 μg per disk decreased the antibacterial activity of amoxicillin, penicillin G and nitrofurantoin while ciprofloxacin activity was enhanced (37). The present study also showed enhanced anti-enterococcal activity of ciprofloxacin, erythromycin, methicillin and vancomycin in combination with ZnO nanoparticles.
Similar activities of all four antibiotics were found with CaO and MgO nanoparticles but at higher concentrations (10 mM). Thus, the MICs of antibiotics conjugates with nanoparticles revealed that the ZnO particles effectively enhanced the MICs of antibiotics in low concentrations in comparison with CaO and MgO nanoparticles.