1. Background
Urinary tract infections (UTIs) are one of the most frequent infections in patients admitted to hospitals (1). Klebsiella pneumoniae are opportunistic bacteria responsible for different infections such as pneumonia, UTIs and septicemia in nosocomial and community environments (2). Beta-lactam drugs that have a beta-lactam ring in their composition are used extensively to save the lives of patients with bacterial diseases (3).
Since the 1980s, beta-lactam antibiotics such as oxyimino-cephalosporins have been used for the treatment of Gram-negative bacterial infections (4). Unfortunately, nowadays, beta lactamase resistance has been growing among members of Enterobacteriaceae, including Escherichia coli and K. pneumoniae. Meanwhile, the most common cause of beta-lactam resistance is beta-lactamase enzymes, which deactivate beta-lactam drugs by breaking down the beta lactam ring (5). Extended-Spectrum beta-lactamases (ESBLs) are a class of enzymes that are encoded by the plasmid, which hydrolyze a wide variety of cephalosporins such as cefotaxime, ceftazidime, ceftriaxone and drugs that have the beta-lactam ring within their structure (6). Extended-Spectrum beta-lactamases were first identified in the early 1980s among Klebsiella species and subsequently in Escherichia coli, Serratia marcescens, Pseudomonas aeruginosa and other Gram-negative bacilli (7, 8).
Infections caused by Gram-negative bacteria producing ESBLs have become a serious problem for hospitals worldwide (9). So far, about 400 different types of ESBLs have been recognized around the world, among which TEM and SHV were more prevalent (10). Mutations occurring in the genes encoding TEM and SHV enzymes lead to the development of ESBLs that have an expanded substrate profile, which allows for the hydrolysis of all cephalosporins, penicillins and aztreonam (11). These enzymes are most commonly produced by Klebsiella spp. Other beta lactamase enzymes are less prevalent (12). VEB can hydrolyze cefotaxime, ceftazidime and monobactams. These enzymes cause durability of various bacterial infections including UTIs in patients (13).
The blaVEB-1-like genes are present as a gene cassette on class 1 integrons that vary in size and structure. In most cases, the VEB-1 cassette has been associated with an arr-2 cassette (rifampin resistance), aminoglycoside resistance gene cassettes, and an OXA-10-like cassette encoding a narrow-spectrum oxacillinase-type beta-lactamase. The blaVEB-1 gene has been reported in several enterobacterial isolates from Asia. Furthermore, VEB-1 ESBL was identified in Vietnam among P. aeruginosa and Enterobacteriaceae isolates (14). Therapeutic options for infections due to ESBL-producing bacteria have also become increasingly limited (8). Phenotypic and molecular detection of ESBL genes such as SHV and TEM can yield appropriate data regarding the epidemiology and risk factors of ESBL-producing bacteria (7, 15).
2. Objectives
Because of the prevalence of beta-lactamase genes in K. pneumoniae isolate and lack of enough information about outbreak of these genes in K. pneumoniae isolates in Shahrekord, this study was conducted to determine the prevalence of TEM, SHV and VEB genes in ESBL-positive K. pneumoniae strains isolated from nosocomial and community-acquired UTIs in Kashani and Hajar university hospitals of Shahrekord, Iran.
3. Patients and Methods
3.1. Data Collection
Over 436 urine samples were collected from different wards (infant, internal, intensive care unit, surgery, emergency, etc.). The isolates were collected from July, 2013 to October, 2014 from two educational hospitals in Shahrekord, Iran. The present study was conducted by the department of microbiology and immunology of the faculty of medicine and cellular and molecular research center of Shahrekord University of Medical Sciences, Shahrekord, Iran. One hundred and fifty isolates of K. pneumoniae bacteria were selected from nosocomial and community-acquired infections in the hospitals under study. Clinical urine samples of patients with UTI were used to isolate bacteria for the study.
3.2. Inclusion and Exclusion Criteria
The patients with a positive urine sample for Klebsiella at admission and history of hospitalization for less than two weeks were considered as outpatients. These patients could be symptomatic or asymptomatic for UTI. However, the patients with primary negative urine culture, who were hospitalized for several reasons rather than UTI (including diabetes, cardiac ischemia, and pregnancy) and whose urine culture grew positive for Klebsiella after 48 - 72 hours, were considered as inpatients. Nosocomial infections are infections developed after patient’s admission to the hospital while the patient should not present UTI symptoms at admission.
3.3. Isolation and Identification of Bacteria
Klebsiella pneumoniae isolates were identified through culture, Gram stain, and microscopy and biochemical standard tests. Blood agar, eosin methylene blue media, and MacConkey agar (Merck Co., Germany) were used for culture. Uropathogenic microorganisms were identified via phenotypic tests of specific culture and typical phenotypic features. Bacterial isolates were then identified by Gram staining (identifying Gram positive or Gram-negative organisms) and a series of standard biochemical tests.
3.4. Antimicrobial Drugs Susceptibility Assay
An antibiotic susceptibility assay was performed via the disk diffusion method (Kirby Bauer), according to the clinical laboratory standards institute criteria (16) on Mueller-Hinton agar plates (Merk Co., Germany). The discs of antibacterial agents used in this study contained amikacin (30 µg), norfloxacin (10 µg), imipenem (30 µg), ceftriaxone (30 µg), ceftazidime (30 µg), cefepime (30 µg), nalidixic acid (30 µg), ciprofloxacin (5 µg), gentamicin (10 µg), nitrofurantoin (300 μg) and trimethoprim-sulfamethoxazole (1.25/23.75 μg). All the antibiotics were obtained from Mast laboratories (MAST, UK). Diameters of the zones of inhibition for individual antibacterial agents were translated into susceptible, intermediate, and resistant categories, according to the clinical and laboratory standards institute (CLSI) criteria (16). Multiple drug resistant microorganisms were identified as resistant to three or more antibacterial classes. Isolates showing inhibition zones of ≤ 27 mm for cefotaxime and ≤ 22 mm for ceftazidime were screened as potential ESBL producers.
3.5. Detection of Extended-Spectrum Beta-Lactamase-Producing Microorganisms
In order to ensure the accuracy of ESBL-producing organisms, K. pneumoniae ATCC 700603 was used as the ESBL-positive and E. coli ATCC 25922 was used as the negative control. The ESBL production was determined using the double disc synergy test. The ESBL-positive strains were detected with phenotypic confirmatory tests using DDST, which contained third generation cephalosporins both with and without clavulanic acid. The discs used contained ceftazidime (30 µg) and ceftazidime + clavulanic acid (30 µg + 10 µg) (MAST, UK). The differences in the zone of inhibition caused by the cephalosporins alone and combined with clavulanic acid were recorded, and if the difference was 5 mm or greater, the strains were confirmed as ESBL-producing.
3.6. Minimal Inhibitory Concentration (MIC) Testing
The MIC for cefotaxime, ceftazidime and ceftriaxone were determined via the E-test (Liofilchem MIC Test Strips, Italy). The E-test has been reported to be a simple and accurate alternative method for determining the antimicrobial susceptibility of various microorganisms. The MIC for cefotaxime, ceftazidime and ceftriaxone was determined, according to the national committee for clinical laboratory standards criteria (16) for phenotypic ESBL-producing K. pneumoniae isolates from UTIs using the E-test strip.
3.7. Preparation of DNA Template for Multiplex Polymerase Chain Reaction
Since most ESBL-producing genes are plasmid-dependent, we used the GeNet bio plasmid kit (plasmid DNA isolation kit, Korea) to extract and purify the plasmid DNA. To extract plasmid DNA, the bacteria were incubated overnight in 5 mL of lysogeny broth at 37°C. Plasmid DNA extraction was based on the GeNet bio plasmid kit protocol. Plasmid DNA concentrations were determined by BioPhotometer (Eppendorf). The extracted DNA was stored under sterile conditions at -20°C (Table 1).
| Antimicrobial Agent | Disk Content, µg | Zone Diameter Interpretive Criteria, mma | MIC Interpretive Criteria, µg/mL | ||||
|---|---|---|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | Susceptible | Intermediate | Resistant | ||
| Ceftriaxone | 30 | ≥ 21 | 14 - 20 | ≤ 13 | ≤ 8 | 16 - 32 | ≥ 64 |
| Cefotaxime | 30 | ≥ 23 | 15 - 22 | ≤ 14 | ≤ 8 | 16 - 32 | ≥ 64 |
| Ceftazidime | 30 | ≥ 18 | 15 - 17 | ≤ 14 | ≤ 8 | 16 | ≥ 32 |
aTo the nearest mm.
3.8. Multiplex Polymerase Chain Reaction for Beta-Lactamase-Encoding Genes
Multiplex PCR for beta-lactamase genes TEM1, SHV1, and VEB1 was performed. Sequences of primers used for the multiplex PCR of the genes are listed in Table 2. The multiplex PCR was optimized according to the following experimental conditions: initial denaturation at 95°C for five minutes, 35 cycles of 94°C for 30 seconds, 56°C for 30 seconds, and 72°C for 40 seconds, followed by a final extension step at 72°C for five minutes and holding a one-time cycle at 4°C for five minutes.
| Primers | Sequences (5' - 3') | Gen Type | Product Size |
|---|---|---|---|
| TEM1 | blaTEM1 | 189 | |
| F | GCTATGTGGCGCGGTATTAT | ||
| R | AAGTTGGCCGCAGTGTTATC | ||
| VEB1 | blaVEB1 | 250 | |
| F | GGGTATTCCAATCCTTGTGC | ||
| R | CCCTCAAGACCTTTTGCCTA | ||
| SHV1 | blaSHV1 | 389 | |
| F | CCTCATTCAGTTCCGTTTCC | ||
| R | CCGCGTAGGCATGATAGAAA | ||
| 16SrRNA | Internal | 420 | |
| F | AGGCCTTCGGGTTGTAAAGT | ||
| R | ACCTCCAAGTCGACATCGTT |
4. Results
The study population of 150 patients with nosocomial and community-acquired infections were divided to two groups of 75 each. An antibiotic susceptibility assay was performed using disc antibacterial agents. The results are shown in Table 3.
| Antibiotic | Concentration, µg | Nosocomiala | Community-Acquireda | ||||
|---|---|---|---|---|---|---|---|
| R | I | S | R | I | S | ||
| AN | 30 | 37 (49.3) | 2 (2.7) | 36 (48) | 23 (30.7) | 1 (1.3) | 51 (68) |
| SXT | 1.25/23.75 | 46 (61.3) | 3 (4) | 26 (34.7) | 39 (52) | 1 (1.3) | 35 (46.7) |
| GM | 10 | 44 (58.7) | 1 (1.3) | 30 (40) | 29 (38.7) | 1 (1.3) | 45 (60) |
| FM | 300 | 41 (54.7) | 5 (6.6) | 29 (38.7) | 24 (32) | 3 (4) | 48 (64) |
| NOR | 10 | 44 (58.7) | 1 (1.3) | 30 (40) | 33 (44) | 1 (1.3) | 41 (54.7) |
| NA | 30 | 54 (72) | 5 (6.7) | 16 (21.3) | 40 (53.3) | 5 (6.7) | 30 (40) |
| CAZ | 30 | 48 (64) | 2 (2.7) | 25 (33.3) | 36 (48) | 2 (2.7) | 37 (49.3) |
| CTX | 30 | 34 (45.3) | 3 (4) | 38 (50.7) | 25 (33.3) | 2 (2.7) | 48 (64) |
| IPM | 30 | 6 (8) | 2 (2.7) | 67 (89.3) | 2 (2.7) | 1 (1.3) | 72 (96) |
| CRO | 30 | 31 (41.3) | 3 (4) | 41 (54.7) | 26 (34.7) | 3 (4) | 46 (61.3) |
| CIP | 5 | 45 (60) | 2 (2.7) | 28 (37.3) | 36 (48) | 2 (2.7) | 37 (49.3) |
aValues are expressed as No. (%).
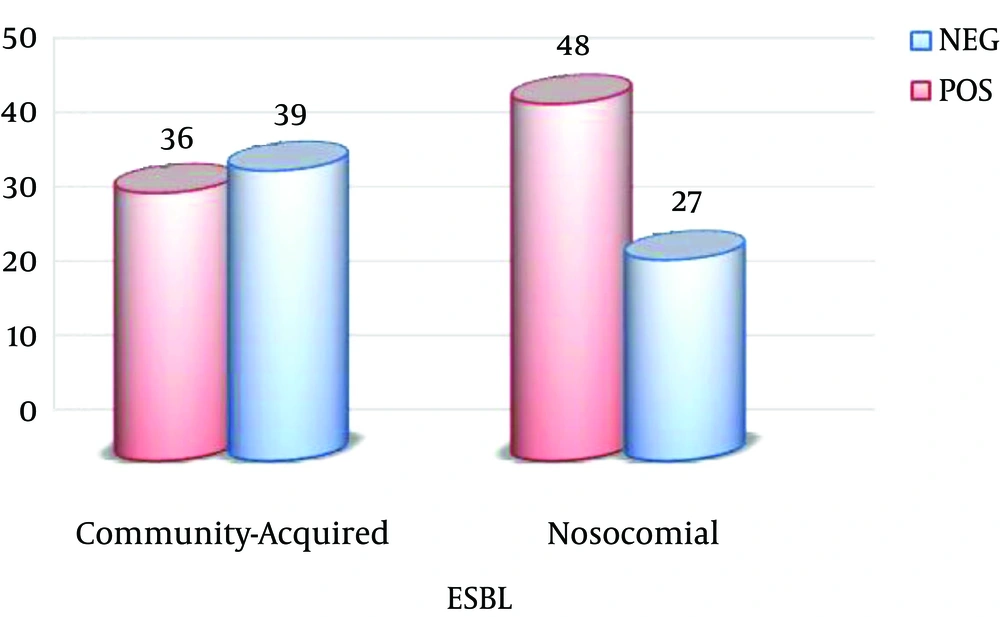
Of the 75 nosocomial samples, at least 54 (71.6%) isolates were resistant to three drug classes. Of these samples, 48 (64%) samples were ESBL-positive. Of the 75 community-acquired samples, 41 (54.9%) isolates were multiple drug resistant. Of these samples, 36 (48%) were ESBL-positive (Figure 1).
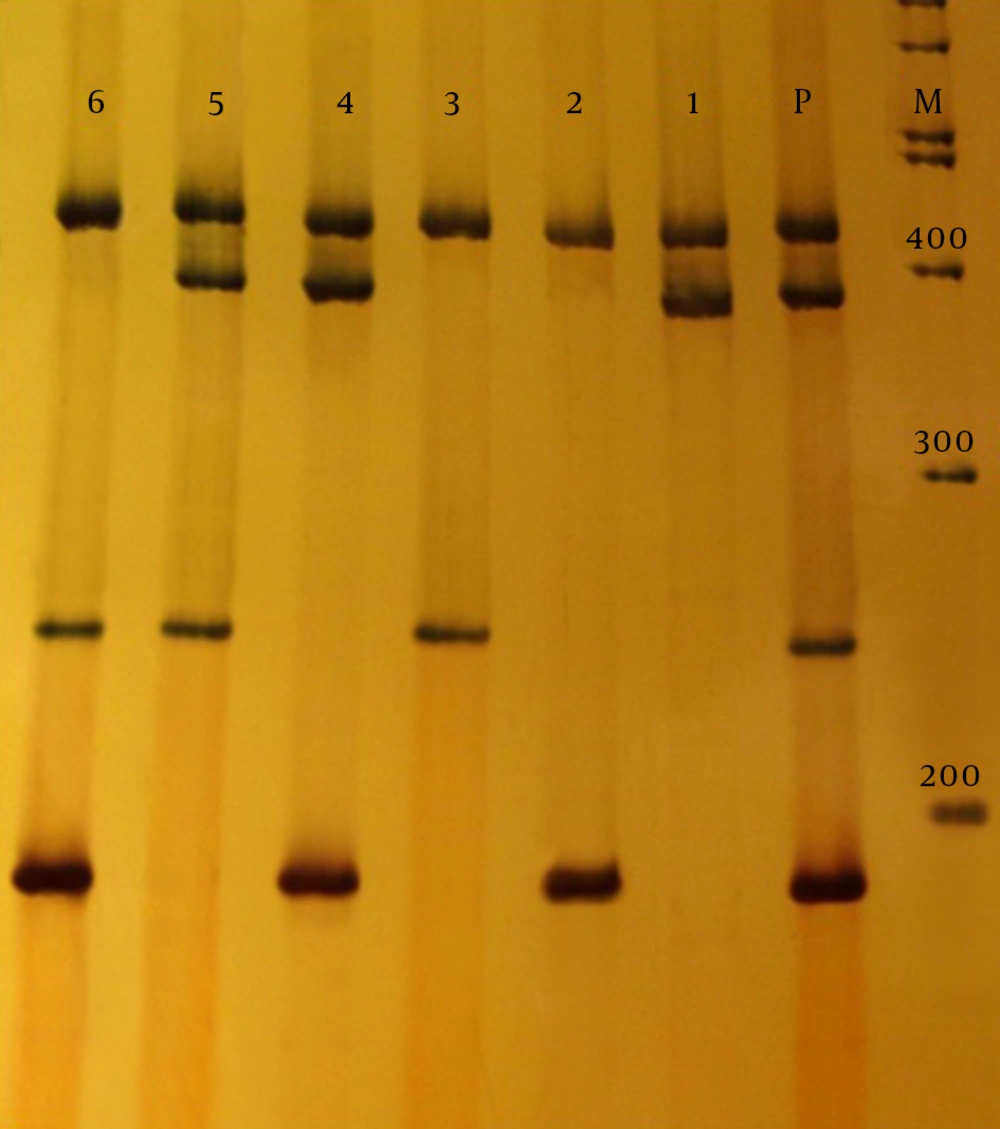
According to the CLSI 2013, in ESBL-producing microorganisms, bacteria isolates were resistant to cefotaxime with MIC ≥ 64 µg/mL and ceftazidime with MIC ≥ 32 µg/mL. The MIC for cefotaxime, ceftazidime and ceftriaxone was determined by E-test strip, according to the CLSI for phenotypic ESBL-producing K. pneumoniae isolates from UTI. In patients with nosocomial infection, resistance of 64%, 45.3%, and 41.3%, and for community-acquired resistance of 48%, 33.3% and 34.7% was obtained for ceftazidime, cefotaxime and ceftriaxone, respectively. Multiplex PCR analysis was performed for beta-lactamase genes TEM1, SHV1, and VEB1 of nosocomial and community-acquired infections. The multiplex PCR products of 189 bp TEM1, 256 bp VEB1 and 389 bp SHV1 were analyzed using electrophoresis on polyacrylamide gels (8%) (Figure 2).
In the nosocomial infection patients, 22 (29.3%) isolates were positive for bla SHV1 gene, 18 (24%) were positive for the bla TEM1, and eight (10.7%) were positive for the bla VEB1. Moreover, four (5.3%) isolates contained both bla TEM1 and SHV1, one (1.3%) contained both blaTEM1 and VEB1, and one (1.3%) contained both bla SHV1 and VEB1. For the community-acquired infections, 17 (22.7%) isolates were positive for bla SHV1, 13 (17.3%) were positive for bla TEM1 and six (8%) were positive for bla VEB1. In addition, one (1.3%) isolate contained both bla TEM1 and SHV1, two (2.7%) isolates contained both bla TEM1 and bla VEB1, and two (2.7%) contained both blaSHV1 and bla VEB1.
5. Discussion
Urinary tract infection is known as one of the most common infections in patients with nosocomial and community-acquired infections (1). Spread of drug resistance factors among Gram-negative bacteria leads to the appearance of strains resistant to antibiotics. These strains cause development of UTIs resistant to antibiotic therapy (17). Study in each area helps to identify the organisms causing UTIs in that area, and determining the resistance of bacteria to antimicrobial compounds that differ in different regions leads to the selection of appropriate treatments for patients (18). The present study investigated antibiotic susceptibility patterns of isolates of K. pneumoniae strains isolated from UTIs in patients with nosocomial infections at university hospitals of Shahrkord, Iran. The lowest resistance to antibiotics in the patients with community-acquired infections was obtained for imipenem (3%) followed by amikacin (31%), and nitrofurantoin (32%). The highest resistance to antibiotics in patients with nosocomial infections was obtained for nalidixic acid (72%), followed by ceftazidime (63%), trimethoprim sulfametizol (61%), ciprofloxacin (60%), norfloxacin (59%), gentamicin (59%) and nitrofurantoin (55%). According to these findings, higher levels of antibiotic resistance were observed in nosocomial infections compared to community-acquired infections.
In a study conducted by Gholipour et al. in Isfahan, Iran, antibiotic resistance to the above antibiotics was lower than that obtained in the present study (6). In another study by Eftekhar et al. in Tehran using antibiotic discs, a higher antibiotic resistance than that obtained in the present study was reported (2). Dallal et al. reported 74%, 59% and 57% resistance to nalidixic acid, ciprofloxacin and ceftazidime, respectively (19), which is in line with the findings of the present study. The MIC of ceftazidime, cefotaxime and ceftriaxone was evaluated, as well. In nosocomial infection patients the resistance of ceftazidime, cefotaxime and ceftriaxone was reported as 75%, 70.8% and 47.9%, respectively, and in those with community-acquired infections, the resistance was reported as 58.9%, 53.8% and 30.7%, respectively. Irajian et al. assessed cefotaxime resistance using the E-test and reported 22.4% resistance for cefotaxime, which is lower than that of the present study (20). In addition, Feizabadi et al. (21) and Mirsalehian et al. (22) reported that the resistance levels to cefotaxime was 84% and 98%, respectively, which is higher than that in the present study.
In a study conducted in Tabriz, cefotaxime resistance was similar to our findings (23). Resistance to cefotaxime using E-test strips has been evaluated in other countries. In a study conducted by Akpaka et al. in Canada, resistance was 44.3%, less than that in the present study (24). Tofteland et al. in Norway reported that 60% of the patients characterized with phenotype ESBLs were somewhat resistant to cephalosporin (25). Following the treatment of UTI with beta-lactam antibiotics, particularly cephalosporins, it is possible for antibiotics to acquire resistance factors with respect to the acquisition by microorganisms (26). Klebsiella pneumoniae is a common cause of urinary infections (27). The main cause of bacterial resistance to beta-lactam antibiotics, particularly various cephalosporins, is the presence of ESBL enzymes in bacteria (28). The prevalence of the bacteria producing ESBLs and resistance to beta-lactam drugs are growing (29). Considering the lack of efficacy of these antibiotics (beta-lactam) in the treatment of infections caused by microorganisms producing ESBLs, it is necessary to assess the antibiotic resistance patterns (30). Here, the prevalence of ESBL in K. pneumoniae isolate strains causing UTI in patients with nosocomial and community-acquired infection was investigated.
Since beta-lactamase genes are derived from TEM1, thus TEM1 and SHV1 as the beta-lactamase genes were studied along VEB1. They are principal factors of hydrolysis compounds such as monobactam and various cephalosporins. The strains of K. pneumoniae-producing ESBLs in nosocomial and community-acquired settings were 64% and 48%, respectively, suggesting a higher prevalence of these enzymes in patients with nosocomial infections for certain reasons such as prolonged treatment procedures and frequent use of urinary catheters during hospitalization. The researches in other regions have reported higher ESBL enzymes prevalence in inpatients than patients with community-acquired infection (31). Zaniani et al. reported ESBL outbreak of E. coli and K. pneumoniae of 43.9% and 56.1%, respectively, in Mashhad (32). Studying K. pneumoniae strains isolated from UTI, Jalalpour reported the prevalence of ESBL in patients with community-acquired and nosocomial infections as 22% and 64%, respectively (33). Therefore, our findings are consistent with his findings regarding the prevalence of ESBLs in ambulatory patients being higher than patients with community-acquired infection, and hence monitoring these patients and identifying ESBL-producing organisms and therapeutic interventions for patients might be promising to prevent outbreaks.
The outbreaks of ESBL-producing K. pneumoniae isolate strains have been reported in various studies. Gholipour et al. reported the prevalence of ESBL-producing K. pneumoniae isolates as approximately 38.1% (6). Feizabadi et al. found that the outbreak of the same isolates was about 44.5% (30). Aminzadeh et al. derived the amount of K. pneumoniae as 52.5% (34) and also Bazzaz et al. reported ESBL-producing isolates of K. pneumoniae and E. coli as 59.2% (35). Mobasherizadeh et al. reported the prevalence of ESBLs in E. coli isolates from nosocomial and community-acquired infections as 64% and 56%, respectively (36). Faizabadi et al., in a study conducted in Tehran on 104 isolates of K. pneumoniae, reported the prevalence of ESBLs as 72.1% (21). In a study by Mirsalehian et al., the prevalence of ESBLs was reported as 59.3% (37).
The prevalence of ESBL enzymes has also been investigated in other countries. In Korea, the prevalence of ESBL was found to be 30% (38). In Pakistan, the prevalence of ESBLs in K. pneumoniae isolates was reported as 36% (39). In a study in India, 68% of K. pneumoniae strains isolated from clinical specimens contained ESBLs-producing isolates (40). Studies have also shown an increase in ESBL-producing strains of K. pneumoniae, including 20% in Algeria (41), 29% in Spain (42), 28.4% in Taiwan (43), and 44% in the USA (44). In light of the above findings, it is clear that the prevalence of a wide variety of ESBL enzymes is rising. It seems that increased and prolonged duration of hospitalization and treatment procedures as well as frequent use of urinary catheters in hospitals are responsible for the emergence of resistant strains and transfer of resistant genes to other bacteria. On the other hand, indiscriminate and arbitrary use of beta-lactam drugs for community-acquired infections causes emergence of resistant strains and their outbreaks.
The majority of ESBL enzymes have appeared upon changes in the structure of TEM and SHV enzymes. These genes are abundant in the Enterobacteriaceae family, especially K. pneumoniae strains (45). The VEB gene is also frequently found among strains of P. aeruginosa and other members of the Enterobacteriaceae family (8). By multiplex PCR in this study, the prevalence of genes SHV1, TEM1 and VEB1 in Klebsiella spp. isolate strains in patients with nosocomial and community-acquired infections was derived 29.3%, 24% and 10.6%, and 21.3%, 16% and 6.6%, respectively. This indicates the high level of expression of these genes in nosocomial rather than community-acquired infections. While the TEM1 gene has already been the most abundant beta-lactamase, several studies conducted worldwide suggest a higher prevalence of SHV1 compared to TEM1 (46). In this study, although a number of isolates were resistant to multiple drug classes, there was a lack of beta-lactamase genes, which could be attributed to the presence of other beta-lactamase genes. This requires further investigation.
According to the present study, the prevalence of beta-lactamase genes in Shahrekord hospitals is high. Therefore, in order to control the spread of resistant strains, appropriate supportive measures, reduction of healthcare costs for patients with UTIs, and further research to determine antibiotic resistance patterns in the region are essential.