1. Background
Lung cancer has occurred in approximately 1.8 million patients worldwide and has caused an estimated 1.6 million deaths in 2016 (1). In the United States, lung cancer occurs in about 225,000 patients and causes over 160,000 deaths annually (2). Lung cancer is the deadliest cancer worldwide and the most common cancer in Asia (3). Helicobacter pylori is a Gram-negative, microaerophilic, slow-growing bacterium, and potent urease activity is its most important biochemical indicator. This bacterium colonizes in the gastric mucosa and causes prolonged local and systematic immune and inflammatory responses in the host. In many developing countries, the prevalence of H. pylori infection exceeds 90% by adulthood; can vary according to age, gender, race, geographical location, and socioeconomic status; and has greater prevalence in developing countries and among blacks (4).
Some recent studies have suggested a relationship between H. pylori and diseases outside the gastrointestinal system, including cardiovascular diseases (5), blood diseases (6), eye and skin diseases (7), hepatobiliary diseases (8), diabetes mellitus (9), and neurologic diseases (10), which suggests that H. pylori may cause a systemic disease. Many studies have demonstrated the presence of H. pylori in upper respiratory system mucosa and the bacterium’s potential role in causing diseases, such as sinusitis, adenotonsillar hypertrophy, pharyngitis, and laryngitis (11-13).
This bacterium is associated with many respiratory diseases, including bronchiectasis, asthma, chronic bronchitis, pulmonary tuberculosis, and chronic obstructive pulmonary disease (COPD) (14-16). Prior to the identification of H. pylori, studies demonstrated a greater incidence of COPD and tuberculosis in patients with peptic ulcers compared with the general population (17). Research has suggested a potential mechanism of the relationship between H. pylori and pulmonary diseases. Since H. pylori is a Gram-negative bacterium, lipopolysaccharide is one of the main compositions of its wall, which stimulates production of proinflammatory cytokines, such as interferon alpha, and interleukins 1, 2, and 8, leading to chronic inflammation, stimulation of the immune system, and cancer (18). Many case-control and epidemiological studies have demonstrated the association between lung cancer with H. pylori. Because of the prevalence and importance of lung cancer and the controversies in some of these studies, the relationship between lung cancer and H. pylori requires further study.
2. Objectives
In the present study, in addition to the immunological assessment of H. pylori in serum samples, the presence of H. pylori in tumor tissue was examined using a urease test, and the bacterium’s presence in bronchoalveolar fluid was examined using PCR.
3. Methods
Patients with suspected lung cancer were enrolled in the study from September 2012 to September 2013 after taking their history, conducting a physical examination, and obtaining their written consent. Permission to conduct this study was obtained from the Ethics Committee of Kerman University of Medical Sciences (No: 255/k/92). Patients unable to withstand bronchoscopy due to cardiac arrhythmias, severe hypoxia, hemodynamic instability, or impaired consciousness were excluded from the study.
The included patients underwent a bronchoscopy conducted by a pulmonologist using a fiber optic bronchoscope (Penthax, Japan). During each bronchoscopy, a tumor tissue sample was taken to be used for urease testing, and 10 cc of bronchial lavage fluid to be used for H. pylori detection using the PCR method was placed in test tubes and stored at -80°C. A 5-mL sample of blood was taken from every patient and stored at -24°C at Afzalipour’s laboratories for use in the serological assessment of H. pylori.
3.1. PCR Protocol
Blood samples were stored at -20°C, and serum samples were used to measure anti-H. pylori IgG using the ELISA method (Triniti kit, Germany). A result of IgG ≥ 1.2 was considered positive. Bronchoalveolar lavage (BAL) samples were collected and stored at -80°C until analysis. These samples were then tested using a genomic DNA extraction kit (Qiacube) according to the manufacturer’s instructions (Boeco, Qiagen-Germany), and the extracted DNA was collected in a 200-µL elution buffer.
Urease primer, the H. pylori urease gene, was used in the Sybergreen PCR test (Table 1) to detect H. pylori. The cagA primer was used to identify pathogenic strains of this bacterium. β-globin gene primers were used as internal controls. The PCR test was considered positive if ≥ 10 copy/mL (19, 20).
| Name of Gene | Forward Primer | Reverse Primer |
|---|---|---|
| ureA | GAGAATGAGATGAAACTCACCC | TTGTCTGCTTGTCTATCAACC3 |
| cagA | CTCATTGCGAAGGCGACCT | TCTAATCCTGTTTGCTCCCCA |
| β-actin | TGCCTATCAGAAAGTGGTGGCT | GCTCAAGGCCCTTCATAATATCC |
The rapid urease test was performed on the BAL samples using the Man Company Kit (Iran) according to manufacturer’s instructions: The biopsy sample was placed in a cup with 1 ml of prepared solution and held for 60 minutes. A sample was declared positive for H. pylori if the color turned from yellow to pink–purple due to production of ammonium by H. pylori.
4. Results
In the present study, the participants’ average age was 60.65 ± 9.15 years. Of the participants, 28 (53.8%) were 40 - 60 years old, 21 (40.4%) were 60 - 80 years old, and 3 were older than 80 years of age. The youngest participant was 40 years old, and the oldest was 86. In terms of gender, 6 (11.5%) were female and 46 (88.5%) were male. Forty-three (82.7%) were smokers, 36 (69.2%) used opium, and 36 (69.2%) baked bread in a wood-burning stove. In terms of clinical symptoms, 9 patients (17.3%) had a hoarse voice, 36 (69.2%) had heartburn, 4 (7.6%) suffered from regurgitation, 2 (3.8%) suffered from dysphagia, and 5 (9.6%) suffered from epigastric pain. In terms of type of lung cancer, 25 (48.1%) had squamous cell carcinoma, 18 (34.6%) had small-cell carcinoma, and 9 (17.3%) had adenocarcinoma.
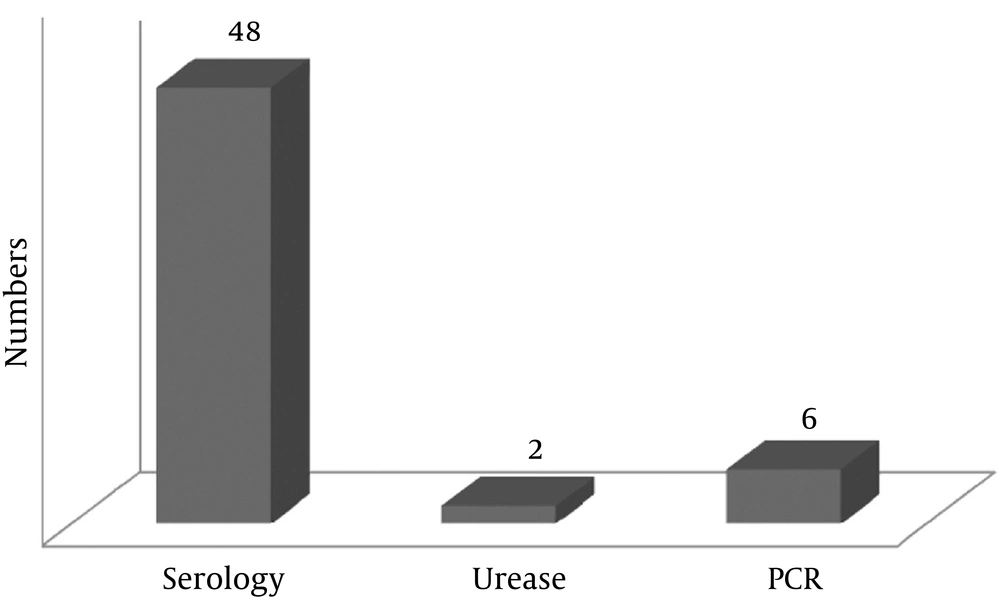
The prevalence of H. pylori in the lung cancer patients was 11.5% (6 patients) according to the BAL PCR test, 92.3% (48 patients) according to the serology test, and 3.8% according to the urease test (2 patients) (Figure 1). Based on gender, the prevalence of H. pylori in the lung cancer patients was 2.2% (a patient) according to the urease test, 13% (6 patients) according to the PCR test, and 91.3% (42 patients) according to the serology test (Tables 2, 3, and 4). The prevalence of H. pylori in lung cancer patients who were smokers was 11.6% (5 patients) according to the PCR test and 90.7% (39 patients) according to the serology test (Tables 2, 3).
| Parameter | Adjusted | Crude | ||
|---|---|---|---|---|
| P Valuea | OR | P Valuea | OR | |
| Sex | 0.999 | 1.01 | 0.999 | 1.53 |
| Ejucation state | 0.673 | 0.795 | 0.570 | 0.826 |
| Job | 0.574 | 0.779 | 0.328 | 0.695 |
| Clinical problem | 0.371 | 0.386 | 0.348 | 0.578 |
| Cigarette smoking | 0.999 | 0 | 0.999 | 0 |
| Opium consumption | 0.536 | 0.348 | 0.795 | 0.773 |
| Wood-burning stove | 0.999 | 0 | 0.999 | 1.40 |
| Age | 0.999 | 0 | 0.998 | 0 |
| Cancer type | 0.494 | 1.34 | 0.759 | 1.10 |
aBased on logistic regression.
| Parameter | Adjusted | Crude | ||
|---|---|---|---|---|
| P Valuea | OR | P Valuea | OR | |
| Sex | 0.999 | 0 | 0.999 | 0 |
| Ejucation state | 0.512 | 1.29 | 0.689 | 0.908 |
| Job | 0.439 | 1.28 | 0.981 | 1.005 |
| Clinical problem | 0.460 | 0.788 | 0.331 | 0.826 |
| Cigarette smoking | 0.990 | 0.971 | 0.965 | 1.05 |
| Opium consumption | 0.126 | 0.005 | 0.885 | 0.875 |
| Wood-burning stove | 0.999 | 2.67 | 0.999 | 0 |
| Age | 0.06 | 0.052 | 0.528 | 0.650 |
| Cancer type | 0.063 | 0.141 | 0.165 | 0.697 |
aBased on logistic regression.
| Parameter | Adjusted | Crude | ||
|---|---|---|---|---|
| P Valuea | OR | P Valuea | OR | |
| Sex | 0.999 | 0.696 | 0.140 | 1.9 |
| Ejucation state | 0.999 | 0.884 | 0.533 | 0.787 |
| Job | 0.999 | 1.44 | 0.506 | 0.819 |
| Clinical problem | 0.999 | 0 | 0.644 | 1.68 |
| Cigarette smoking | 0.999 | 2.26 | 0.997 | 0 |
| Opium consumption | 0.999 | 0.075 | 0.558 | 0.429 |
| Wood-burning stove | 0.999 | 0.329 | 0.025 | 9.4 |
| Age | 0.999 | 1.56 | 0.277 | 0.304 |
| Cancer type | 0.999 | 8.62 | 0.969 | 1.02 |
aBased on logistic regression.
The prevalence of H. pylori in lung cancer patients according to type of cancer showed that the urease test was negative in all patients with squamous cell carcinoma and adenocarcinoma and that only two patients (11.1%) with small-cell carcinoma were positive for H. pylori. No significant relationship was found between H. pylori and type of cancer according to the urease test (P = 0.969) (Table 4). The BAL PCR test was positive in one patient (4%) with squamous cell carcinoma, three patients (16.7%) with small cell carcinoma, and two patients (22.2%) with adenocarcinoma (P = 0.165) (Table 3). The serology test was positive in 32 patients (92%) with squamous cell carcinoma, 17 patients (94.4%) with small-cell carcinoma, and 8 patients (88.9%) with adenocarcinoma (P = 0.759) (Table 2).
5. Discussion
Unexpectedly, 11.5% of patients with lung cancer were positive for H. pylori according to the BAL PCR test, 92.3% were positive according to the serology test, and 3.8% were positive according to the urease test. In early studies on the prevalence of H. pylori, 90% of cases were positive according to serology testing (21), which is similar to the results of the present study. A survey from Nigeria reported higher values: The prevalence of H. pylori was 80% when tested with histology and was even higher when serology was applied, reaching 93.6% (22). One study conducted in Iran reported the prevalence of H. pylori in patients with lung cancer using the ELISA method to be 73% (23). In another study on patients with adenocarcinoma and squamous cell carcinoma in the lungs, the prevalence of H. pylori was reported to be 79.7% (24). In a study that was conducted at Tehran University of Medical Sciences, the prevalence of H. pylori in patients with lung cancer was reported to be 52.2% according to serology testing (25). A similar result was obtained in Greece, with the prevalence of H. pylori being reported as 61.1% (26).
These studies were all case-control studies that were based on serology testing, and there are many confounding factors in serology testing. For example, Iran is considered to be a high-prevalence region in terms of H. pylori infection, and almost 90% of adults are infected with this bacterium (27). Moreover, factors such as age, gender, socioeconomic status affect H. pylori infection. Additionally, smoking is a confounding factor and is one of the main causes of lung cancer.
The association between H. pylori and smoking remains controversial. Since the majority of people with lung cancer are smokers, and despite the believed association between H. pylori infection and smoking, one study revealed that H. pylori was negative in patients with lung cancer according to serology testing, and in a five-year follow-up, no association was found between smoking and H. pylori infection (28). In another study, 72.7% of control subjects were smokers (smoking more than four daily cigarettes during the last year), but H. pylori seropositivity was significantly lower in the control group than the case group. Therefore, H. pylori alone, independently from smoking, might be a risk factor for lung cancer (23). In the present study, no significant relationship was found between H. pylori and smoking.
Given the common embryonic origin of respiratory and gastrointestinal systems and due to neuroendocrine and paracrine processes, hormones such as gastrin are secreted, which are the strongest mucosal proliferation factor in the lung and gastrointestinal system and which cause chronic inflammation by inducing COX-2. Studies have demonstrated that lung cancer tissue and resection margins have contained amounts of immunoreactive gastrin many times larger than intact bronchial mucosa (21). Similarly, another study revealed that serum gastrin level is a useful predictor of response to chemotherapy and survival in patients with small-cell lung cancer (29).
The treatment of H. pylori has been greatly emphasized in lung cancer patients to reduce the stimulation of gastrin production (30). Recently, research of indirect evidence has suggested a possible association between H. pylori and pulmonary disease. This study aimed to determine whether H. pylori could be detected in endobronchial specimens collected from patients undergoing a bronchoscopy.
In one study, 34 patients with a variety of pulmonary diseases underwent a bronchoscopy and biopsy of lung tissue. Samples were examined in terms of histopathology and urease testing, and no result was found in the biopsies or urease tests in favor of H. pylori. In the present study, the urease test was positive in 3.8% of patients. In another study on bronchiectatic patients, BAL fluid and lung tissue were examined for H. pylori PCR, but bacterium DNA was not found in any of the patients. In the present study, H. pylori DNA was found in 11.5% of patients in BAL fluid using the PCR method. In a recent study in Kerman on 60 patients with COPD, H. pylori was found in 10% of patients using PCR testing, in 88.3% of patients using serology, and in 0% of patients using urease testing (16). These results are similar to those of the present study.
The lack of a control group was one of the present study’s limitations. Since bronchoscopy is an invasive method, use of healthy people in a control group was ethically wrong. Another limitation was the study’s small sample size, which made it impractical to assess the possible relationship between smoking and H. pylori based on lung cancer histopathology. Thus, a similar study with a larger sample size is recommended. We unexpectedly detected H. pylori DNA in 11.5% in the BAL fluid of lung cancer patients using a real-time PCR method for the first time. Most studies have been based on serology testing. In addition to serology testing, a real-time PCR method was used to detect this bacterium. The aim of this study was to investigate the possible association between H. pylori infection and lung cancer via the local inflammatory response in the airway, direct damage and chronic inflammation through inhalation and aspiration, and the systematic immune response induced by H. pylori colonization. This local and systemic inflammation is assumed to be caused by extracellular products excreted by H. pylori together with the genetic predisposition of the host and other environmental risk factors that predispose an individual to lung cancer. This association requires further investigation by well-designed prospective studies. Future studies on patients with lung cancer should examine genetic loci by identifying HLAs that can predispose the individual to both H. pylori infection and lung cancer.