1. Background
Leishmaniasis is a life-threatening disease that is caused by a protozoan parasite of the genus Leishmania; this genus is composed of more than 20 different species. The disease is transmitted through phlebotamine sand flies, which consist of more than 30 species in old-world leishmaniasis and Lutzomia in terms of new-world leishmaniasis (1). The life cycle of Leishmania is digenetic, alternating between motile, extracellular, and flagellated promastigotes. It develops in the vector gut and is non-flagellated. Non-motile amastigotes form and are grown in a mammalian host (2). Leishmaniasis has been categorized into three different clinical forms: cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis (MCL), and visceral leishmaniasis (VL), all of which vary in terms of their immune pathologies and the extent of mortality and morbidity (3). Globally, the disease affects 12 million people. Incidences of 0.5 million new cases of VL and 1.5 million cases of CL are reported annually (4). There has been a 42-fold increase in the prevalence of leishmaniasis over the last 15 years (5).
Variations in molecular and biochemical characteristics reveal the variable susceptibility of Leishmania to drugs, such as pentavalent antimonials, paromomycin, azoles, pyrazolopyrimidines allopurinol, and allopurinol riboside (6). The factors involved in the increase in incidents include the lack of effective control measures for both vectors and parasites, poor knowledge of the disease, a lack of health policies, and the suboptimal dosage of the drug, which has been increasing rapidly (7, 8). Glucantime is the first line of treatment for leishmaniasis. A resistance of 50% to glucantime has been reported in India (9). P-glycoprotein-mediated resistance in Leishmania to ether lipids has already been identified in Leishmania tropica (10).
Amphotericin B (amp B) is a polyene antibiotic that is used as the second line of treatment for CL. Amp B binds to ergosterol, the main sterol present in L. tropica, Trypanosome cruzi, and fungi. Lipid formulations (AmBisome and Amphocil) of amp B have been developed to overcome toxicity and resistance, as well as to improve the therapeutic index. They represent an alternative treatment for CL and VL (11-13). The sensitivity of Leishmania to available and novel drugs has significant clinical implications.
2. Objectives
As amp B formulations are increasingly used for the treatment of leishmaniasis, we decided to study the sensitivity of itraconazole, ketoconazole, and miltefosine that are resistant, as well as the sensitivity of wild forms of the L. tropica strain.
3. Materials and Methods
3.1. Materials Used
Media M199 (Invitrogen, USA), RPMI (Invitrogen, USA), penicillin (Sigma, Germany), Streptomycin (Amresco, Ohio), a 96 well plate (SPL Life Sciences, Korea), fetal bovine serum (PAA Laboratories GmbH, Austria), amp B (Sigma-Aldrich), miltefosine (Sigma-Aldrich), ketoconazole (Sigma-Aldrich), itraconazole (Sigma-Aldrich), and a micropipette (Eppendorf) were used in the experiment. The other chemicals were of an analytical grade and were used as such, without further purification.
3.2. The Attenuation of L. tropica
The infective culture of the L. tropica strain was supplied by the parasitology research laboratory of the Department of Zoology, University of Peshawar, Khyber Pakhtunkhwa, Pakistan. The culture was maintained in a M199 medium containing 10% heat-inactivated fetal bovine serum (hiFBS) at 24°C. Every 5 days, the strain was subjected to continuous passaging 20 times (13). After the first subculture was treated, the parasites were inoculated intradermally to a group of six BALB/c mice (purchased from the national institute of heath, Islamabad, Pakistan). The animals received humane care in accordance with the guidance on the operation of the animals (scientific procedures) Act of 1986 (14). The attenuation of the culture was confirmed by inoculating the parasite in the BALB/c mice. The CL lesions in the mice were checked every 2 days for a period of 30 days.
3.3. The Development of the Resistant Strain
The resistant line of the original clone of L. tropica was produced according to the procedure previously described (15). The wild-type culture was grown in M199 containing 10% hiFBS. The culture was continuously monitored, and passaging was carried out every 5 days. A step-by-step process was started in which the culture was initially incubated with 0.01 µg/mL of amp B for 7 days and subsequently exposed to 0.02, 0.03, 0.04, and 0.1 µg/mL. The drug concentration increased accordingly. The promastigotes showed a growth rate equivalent to that of the wild-type clone cultures. This process was carried out for four months. The resistant lines that were produced were stored at 24°C. The new, attenuated amp B-resistant L. tropica strain was cryo-preserved at -196°C in liquid nitrogen.
3.4. The Stability of the Resistant Strain
To monitor its stability, the resistant strain was grown in the M199 medium without amp B. LC50 was determined every week for three months.
3.5. Determining the Resistance Level
After the successful production of the resistant strain of L. tropica, the culture was treated with different concentrations of amp B in a two-fold dilution series. The promastigotes (106 cells/mL) were exposed to amp B for 24 hours. The percentage of survivors of the promastigotes was calculated via sigmoidal analysis using SPSS version 21.
3.6. Cross Resistance
Stock solutions of ketaconazole, itraconazole, and miltefosine were prepared by dissolving 20 mg in 1 ml of DMSO. L. tropica strains in the logarithmic phase were harvested and diluted to concentrations of 1 × 106 cells/mL. The culture was incubated with similar drugs for 24 hours. Eight different concentrations in two-fold dilutions were used in the experiment.
3.7. In Vitro Antileishmanial Assay
3.7.1. Culture Preparation
L. tropica was cultured in M199 media containing 10% hiFBS. The culture was incubated at 24oC and was passaged every 7 days.
3.7.2. Assay Procedure
An in vitro anti-promastigotes assay was produced according to the procedure previously described (16). Briefly, 1 × 106 promastigotes from L. tropica in the logarithmic phase were harvested and inoculated on a 96 well plate containing M199 media. Stock solutions of 10,000 ppm were prepared as the test samples. The experiment began at a high concentration of 100 ppm and was serially diluted. The experiment was carried out in triplicate. The plates were incubated at 24°C for 48 hours. After this specified period of time, 15 µL of the culture was placed on a Neubauer counting chamber and was examined under a light microscope (magnified to 40x). The percentage of the survivors from the treated and untreated culture was recorded. The data were then processed using SPSS version 21 software (IBM, New York, USA) for LC50 calculations.
3.8. Statistical Analysis
All of the experiments were carried out in triplicate. The data obtained were statistically analyzed using SPSS version 21 software. For comparison, post hoc multiple comparison tests were performed. The values were represented as means ± the standard deviation. A probability value of P < 0.05 was considered significant.
4. Results
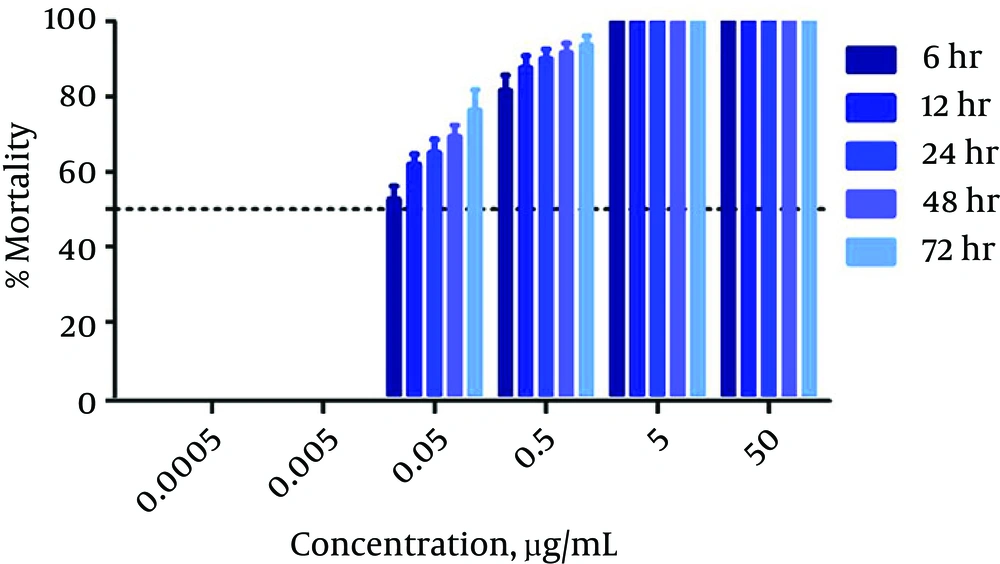
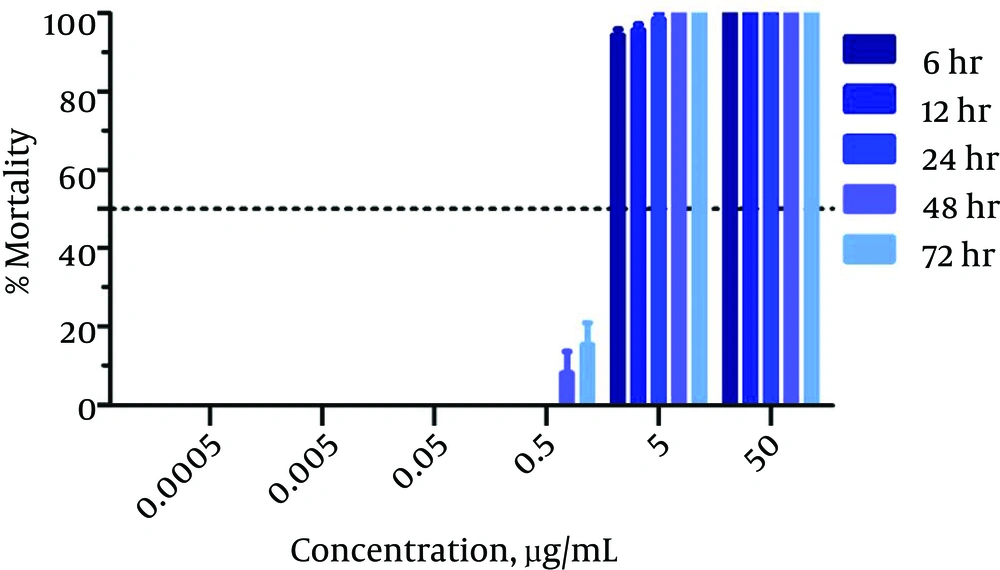
An in vitro anti-promastigotes assay was created for use against wild and resistant-type L. tropica strains. The activity was observed at different time intervals (6 - 72 hours), as shown in Table 1. Amp B exhibited significant activity against the L. tropica strain. The LC50 value calculated for amp B was 0.023 µg/mL after 72 hours of incubation. The percentage of mortality among the parasites for amp B is presented in Figure 1. The time-drug effect of amp B on the resistant strain was investigated. After 72 hours of incubation, the LC50 value was calculated as 0.432 µg/mL. The percentage of mortality of the L. tropica resistant strain is shown in Figure 2.
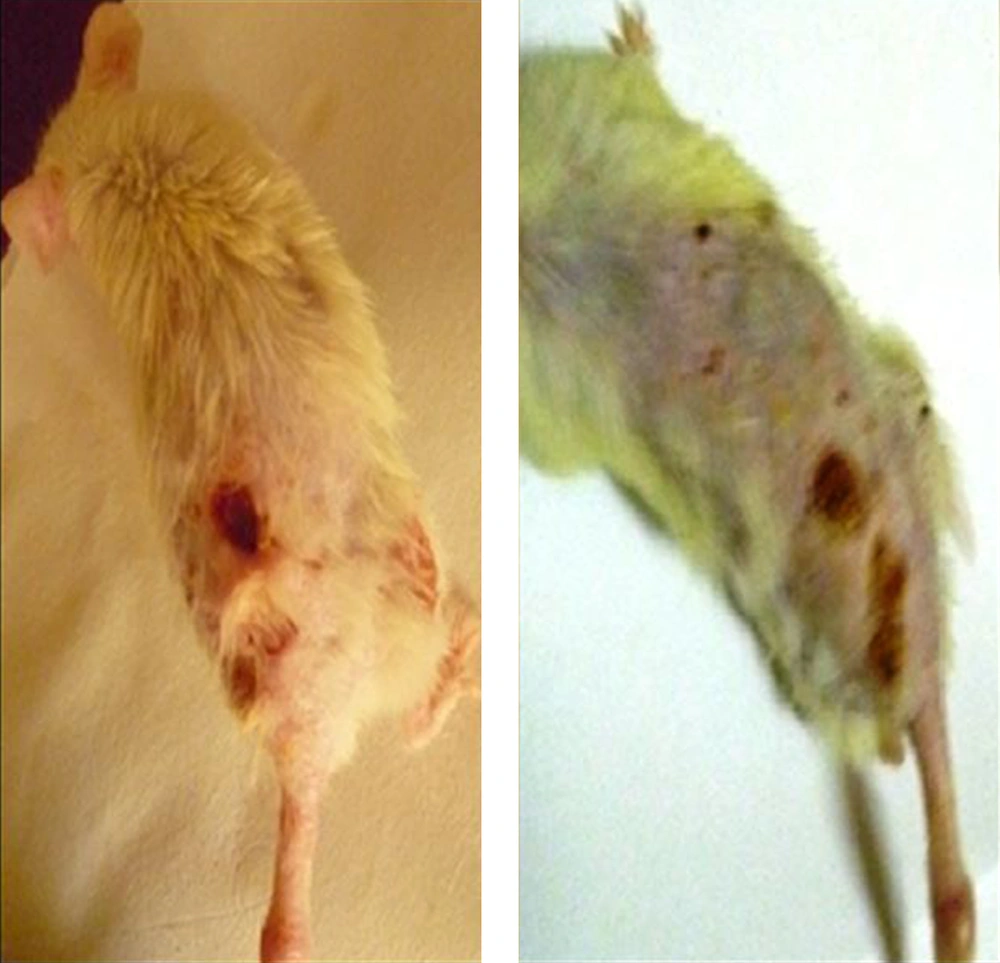
To provide the attenuation of L. tropica, six mice were inoculated with the wild type of the infective Leishmania culture. Lesions appeared after two to three weeks at the site at which the parasite was injected (the base of tail; Figure 3). The lesions were characterized by swelling, stiffness, and redness at the onset of the disease. Two weeks later, nodules, papules, and plaque formed, which transformed into an ulcer that increased in size over time. Later, L. tropica was continuously passaged 20 times. After 20 passages, L. tropica was unable to cause infections in the BALB/c mice. There were no CL lesions observed for the wild type of the Leishmania parasite.
| Time, h | Antileishmanial Activity of amp B, LC50 µg/mL | |
|---|---|---|
| Wild Type | Resistant Type | |
| 6 | 0.037 | 0.623 |
| 12 | 0.035 | 0.595 |
| 24 | 0.030 | 0.551 |
| 48 | 0.025 | 0.543 |
| 72 | 0.023 | 0.432 |
The Antileishmanial Activity of amp B at Different Time Intervals Against Wild and Resistant Types of L. tropica
The amp B-resistant L. tropica strain was developed from the attenuated wild type using a continuous stepwise increase in drug pressure. L. tropica was initially exposed to 0.01 µg/mL of amp B. A total of 6 passages were completed from 0.01 µg/mL to 0.02 µg/mL in concentration. Subsequently, the concentration level was raised from 0.02 to 0.03 µg/mL for the next 3 weeks. Four passages were performed. At a concentration of 2 µg/mL or higher, no growth was observed. The sensitivity of the resistant strain was indicative of a 16-fold increase in resistance to amp B, as shown in Table 2. The resistant strain of L. tropica was investigated for cross resistance to other antileishmanial drugs. The LC50 values were established for the drugs that were to be used against the wild and resistant strains in a 72-hour assay (Table 3). Among the drugs (itraconazole, ketoconazole, and miltefosine), L. tropica showed resistance only to itraconazole, with a resistance index measuring greater than 5 (Table 4). The stability of the resistant strain was confirmed by determining the LC50 values, which were within a similar range as those that were initially identified (Table 5).
| Amp B | Wild Type | Resistant Strain |
|---|---|---|
| LC50, µg/mL | 0.031 | 0.501 |
Sensitivity of the Wild and Resistant Strains of L. tropica
| Drugs | Cross Resistance Indicesa |
|---|---|
| Resistant Strain | |
| Itraconazole | 5.87 |
| Ketoconazole | 1.06 |
| Miltefosine | 1.08 |
Cross-Resistant Indices for the Resistant L. tropica Strain
| Drug | LC50, µg/mL | |
|---|---|---|
| Wild Type | Resistant Strain | |
| Amp B | 0.021 | 0.435 |
The Stability of the Resistant L. tropica Strain
5. Discussion
The resistance of Leishmania to different drugs is a hot topic. The ability to overcome this resistance is a great challenge for scientists, physicians, and for those in the pharmaceutical industry. As part of study on the antileishmanial activity of amp B, ketoconazole, itraconazole, and miltefosine, amp B-resistant promastigote lines were selected by steadily increasing the drug pressure, as previously shown using other antileishmanial drugs (17-20). The resistant form of the parasite was generated to a maximum concentration of 0.1 µg/mL in the M199 medium. The parasite was unable to grow at a concentration of 0.2 µg/mL. This type of resistant line exhibited LC50 values for amp B that were 16 times higher than those of the wild type. This is because Leishmania parasites amplify certain parts of their genomes in response to stress, such as drug pressure (21, 22); this plays a key role in drug resistance. Cross resistance was determined and limited to itraconazole, an azole antifungal drug with antileishmanial activity against L. Mexicana (23). Past or concurrent treatment with an azole might lead to amp B resistance (24). The proposed mechanism might undergo a change in the sterol type or in the content of the surface membrane.
Resistance to polyene can be attributed to the alteration of ergosterol biosynthesis (25). Kelly et al. (26) observed cross resistance to amp B in two fluconazole-resistant strains of Candida albicans that were isolated from patients with AIDS. Ketoconazole and miltefosine exhibited ideal antileishmanial activity against wild and resistant strains, and no cross resistance was observed. The time-dependent activity of the antileishmanial drug has been previously reported. This variation in activity is not easy to interpret, as it might result from differences in the division rate, the exposure to extracellular and intracellular phases of the drugs, the metabolism of the drug, or the biochemical targets (27).
In the metacyclic stage, the Leishmania parasite is more infectious and causes infections in their host after deposition into the dermis. There are several virulence factors responsible for infection progression, including glycosylphosphatidylinositol (GPI), glycosilinositolphospholipids (GIPLs), cysteine protease B (CPB), lipophosphoglycan (LPG), and zinc-metalloprotease GP63 (GP63) (27, 28). The virulent culture of L. tropica when inoculated into the BALB/c mice was observed. An infection developed in four mice after two weeks of incubation. The culture lost infectivity after 20 passages, and its attenuation was confirmed through the inoculation of the BALB/c mice. This was in agreement with the findings of Ali et al. (13). The loss of infectivity of the microorganism due to in vitro culturing is well established, and the production of attenuated strains of the pathogenic microorganism has proven to be safe after its use as a vaccine (29).
A number of studies have reported on the resistance of promastigotes to antileishmanial drugs because the resistant strain is easy to produce and study. The study of resistant strains of a particular parasite is the best approach to find solutions to a particular drug-resistant regime.