1. Background
Herpesviridae is a family of viruses with double-stranded DNA. The B virus (Cercopithecine herpesvirus 1, or herpes B virus) and Cercopithecine herpesvirus 2 (CeHV-2) are primate herpes viruses that belong to the alpha-herpesvirus subfamily, and are closely related to herpes simplex virus 1 (HSV-1). The coated particle harbors a linear double-stranded DNA genome of about 157 kb (1-4). Herpes B virus (BV) is a zoonotic virus and its natural host is mainly cercopithecidae, although BV infections are usually mild or unapparent in macaques, yet lead to fatal encephalitis and encephalomyelitis in humans (2, 5, 6). Previous studies (7) have shown that BV is the only known agent that could infect humans among the 35 herpesviruses confirmed in nonhuman primates. In the early stages of infection, if not treated by antiviral therapy, the infection would have a high mortality.
As nonhuman primates are the main experimental animals for biomedical research, development of an effective method for surveillance of BV infection is essential for the establishment of BV-free monkey colonies and reduction of the risk for laboratory workers, animal handlers and researchers (8). Unfortunately, in most cases, usage of cell culture or polymerase chain reaction (PCR) for an immediate diagnosis of BV infection was impossible, for the existence of similarity between BV and other alphaherpesviruses. Herpes B virus spends a life-long lurking in sensory ganglia of macaques and rarely reactivates (9). The detection of serum antibodies to BV proteins was used for diagnosis of BV infections in humans and monkeys. Thus, serological test of anti-BV antibodies is the only effective way for the surveillance of infected animals.
Currently, a few serological test techniques are being used to detect the viral infection, relying on recombinant proteins and cell lysates of BV (10). To identify infected animals, the solubilized HSV-infected cell antigen was developed and used for ELISA and other rapid serological tests (11-13), with subsequent western-blotting confirmation to identify specific targets that could be immunoreactive with serum antibodies (14). Because of high cross-reactivity with BV proteins, HSV type 1 (HSV-1) and HSV-2 are not specific enough to clearly identify BV infections in herpes simplex virus (HSV) positive humans by this method (15, 16). Among the twelve glycoproteins, which contained in the viral envelope, four had proven with high immunogenicity, gB, gD, gC and mgG (3, 10, 17), the primary targets of IgG antibody responses of the in patients, who were infected by the HSV-1 or HSV-2 have been confirmed as the nucleocapsid complex p40, and the major capsid protein VP5 (6, 16, 18-20), it would be a complex task to differentiate them.
Most importantly, BV is a biosafety level 4 (BSL-was similar with glycoprotein D of HSV-1 and4) pathogen; currently BV-infected cell lysates are used as a diagnostic antigen in serological tests and only maximum containment laboratories (BSL-4) have the qualification to produce it, this requirement limits the amount of facilities, which provide the antigen. When compromising outcome measures based on assays using these antigens, the antigens may also suffer from lot-to-lot variation. The glycoprotein D (gD) of BV is a 35-kDa glycoprotein identified in the BV envelope and is among the main surface antigens shown to elicit antibodies in sera of infected animals.
It has a high homology with HSV-1; its amino acid sequence is 56% - 58% identical to that of HSV-1 gD (1). Moreover, BV gD was similar with glycoprotein D of HSV-1 and HSV-2, and is one of the immunodominant envelope proteins that can elicit humoral and cellular immunity in animals and human individuals; the gD protein has been considered as a good immunogen (6, 21). Finally, BV gD protein could bind to human nectin-1 receptors for cell-cell fusion and virus entry (22, 23). Therefore, in B virus infection the gD has been identified as a multifunctional protein, and production of mAbs against gD could also provide an effective tool for elucidation of the exact function of BV glycoprotein gD.
2. Objectives
The aim of the present study was to prepare anti-BV mAbs, which is for the diagnosis of BV infection, and provide a foundation for the development of rapid and specific methods.
3. Materials and Methods
3.1. Cell Lines
The mouse myeloma cell line, SP2/0, was cultured in RPMI-1640 medium supplemented with 10% fetal calf serum (Gibco, USA) at 37°C in a humidified atmosphere in the presence of 5% CO2 (24). Escherichia coli Rosetta (DE3) competent cells were prepared using the calcium chloride method and stored at -80°C (25).
3.2. Optimization of gD Gene and Construction of Its Expression Vector
The nucleotide sequence for envelope glycoprotein D gene of BV strain E2490 has been deposited in the GenBank database and the gD gene was optimized with the following accession number, NC-004812. The transmembrane region of gG was analyzed by the SOSUI system, as described previously (26). The extracellular domain of viral glycoprotein usually is the key point for eliciting host antibodies. Therefore, the extracellular region of gD was chosen for codon optimization and synthesized. The EcoR I and Hind III restriction endonuclease (TaKaRa, Japan) sites in the sequence were then introduced to allow ligation of the entire gD sequence into pET-32 (+) (Novagen, Germany); an amino-terminal histidine tag contained the prokaryotic expression vector.
3.3. The Expression and Purification of gD Recombinant Proteins
DNA sequencing and restriction endonuclease digestion were used to verify the synthesized gD gene before expression. The recombinant plasmids were transformed into E. coli Rosetta (DE3) cells, and E. coli cells were grown in shaker flasks with LB broth medium containing 100 μg/mL ampicillin at 37°C until optical density (OD) of 0.6 was reached, and then recombinant bacteria were induced using 0.1 M of isopropy-Β-D-thiogalactoside (IPTG) at 37°C for four hours. The induced recombinant bacteria were analyzed by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (27). Four hours after induction, the cells were harvested and centrifuged at 9,000 rpm for ten minutes and washed once with phosphate buffered saline (PBS). Subsequently, the cells were subjected to rapid freeze-thaw treatment at -80°C twice and resuspended in PBS buffer. In addition, cell membranes were disrupted by supersonic waves, and precipitated. The supernatants were collected at 9,000 rpm for 10 minutes at 4°C.
The extracted inclusion body proteins and supernatants were treated with 10 × SDS loading buffer and then verification by 12% SDS-PAGE. Inclusions were processed with urea of 8 M at 4°C cracking overnight. The recombinant His-tagged gD was purified with a column of nickel-nitrilotriacetic acid (Ni2+-NTA) agarose resin. Cell pellets harvested from LB broth were resuspended in 20 mL of PBS. The cell pellets were then sonicated for 15 minutes and centrifuged at 12,000 g for 15 minutes. The inclusion bodies were solubilized in buffer A (8 M urea in ddH2O) by incubating the solution at 4°C for eight hours.
The solubilized solution was centrifuged for 20 minutes at 12,000g at 4°C. The supernatant was applied to Ni2+-NTA column. The unbound proteins were removed by washing buffer (20 mM Tris-HCl, 500 mM NaCl, 5 mM imidazole, pH 7.9, 8 M urea; one times 6 mL). The elution of gD was accomplished with elution buffer (20 mM Tris-HCl, 500 mM NaCl, 200 mM imidazole, pH 7.9, 8 M urea; five times for 5 mL each). Finally, protein elution solutions were dialyzed against PBS (pH 7.4) for six hours. Protein concentration was measured by standard BCA assay (Cowin Biotech, Beijing, China) according to the manufacturer's instructions, and proteins were aliquoted and stored at -80°C (28).
3.4. Western Blot Analysis
To verify the recombinant gD protein expressed in E. coli Rosetta (DE3) cells, western blot analysis was performed using the following procedures (29). Three samples of the purified gD protein were subjected to 12% SDS-PAGE and then transferred to polyvinylidene fluoride (PVDF) using a wet transfer apparatus (Bio-Rad). After blocking with PBS containing 0.05% tween-20 (PBST) and 5% skimmed milk for two hours, one of the PVDF membranes was added to the mouse anti-His-tag mAb (Cwbio, Beijing, China) at a dilution ratio of 1:5000 in 5% skimmed milk solution at 37°C for two hours. After washing with PBST, goat anti-mouse IgG conjugated horseradish peroxidase (Santa Cruz Biotechnology, CA) as secondary antibody, was added at a dilution ratio of 1:3000 and incubated at 37°C for one hour, and then incubated with DAB solution (Cwbio, Beijing, China) for 5 - 10 minutes. The second PVDF membrane was added to monkey B virus serum at a dilution ratio of 1: 500 in 5% skimmed milk solution and the third one was added to monkey negative serum at the same dilution ratio, both of them used the goat anti-rhesus IgG (H + L) HRP (Southern Biotech, USA) as a secondary antibody, this procedure was followed by eECL western blot analysis (Cwbio, Beijing, China). The cell culture of pET-32a-gD-Rosetta (DE3) without IPTG induction was used as the negative control.
3.5. Immunization and Generation of Hybridomas
Three six to eight week-old adult BALB/c mice with body weights of about 17 g were purchased from the experimental animal center of Kunming Medical University. For the initial immunization, three BALB/c mice were immunized with 1:1 mixtures of gD recombinant protein (25 μL of protein per mouse with the addition of PBS to reach a final volume of 150 μL) and complete Freund’s adjuvant (Sigma, St. Louis, MO). Pre-immune serum was collected prior to immunization as the negative control. The first two injections were on both sides of the subcutaneous tissue of the back and neck. The last boost was into the intraperitoneal region. Two weeks after each immunization, a blood sample was obtained from the tails of the immunized mice and tested for titers against gD by ELISA. Recombinant protein was used to coat ninety-six-well plates with a concentration of 0.35 μg/well. Each serum sample was diluted from 1:500 to 1:128,000 by 2-fold serial dilutions.
The secondary antibody was HPR-labeled goat-anti-mouse IgG. A positive OD (OD for positive well) > 0.1 and OD positive / OD negative (OD for negative well) ≥ 2.1 were the criteria for positive results. The mouse with the highest serum titer was selected for fusion, whose spleen was removed and splenocytes were fused with the mouse myeloma cell line (SP2/0). According to previous research, 3:1 - 10:1 were the ratio of the spleen cells mixed with SP2/0 cells. After centrifugation, 0.8 mL of 50% PEG1450 was added drop by drop over one minute into mixed cells and after 90 seconds in order to stop the reaction, serum-free RPMI-1640 medium was added. After centrifugation, the fusion cells were resuspended in RPMI-1640 selective medium containing 20% FBS and 1% HAT, and 100 μL/well of this solution was added to 96 well plates already loaded with the feeder cells. The incubation conditions were 37°C and 5% CO2. After fusion, when hybridoma cells covered 1/3 of the area of culture wells, indirect ELISA using gD as coating antigen was screened for individual hybridoma clones in the culture supernatant, using the SP2/0 cells culture supernatant as the negative control (13, 30).
Cells in antibody positive wells were sub-cloned in accordance with the limiting dilution method (30). Positive hybridoma cells were transferred from 96 well plates to 24 well plates, and then transferred to six well plates after overgrowing, and were finally transferred to cell culture flasks for incubation. Incubated cells were cryopreserved. The cell culture supernatants were collected for titer detection by ELISA and were preserved at -80°C. Prior to commencement of the study, all experimental protocols that involved animals received approval from the scientific and ethical committee of the Kunming University of Science and Technology (PR. China).
3.6. Preparation and Purification of Ascites
Scalable culture of anti-gD antibody-positive hybridoma cells was conducted. Firstly, six to eight week-old female BALB/c mice were injected intraperitoneally with 0.5 mL of sterile liquid paraffin. One week later, each BALB/c mouse was injected intraperitoneally with 5 × 104 hybridoma cells in 0.5 mL PBS. Ascites of mice with extremely enlarged abdominal circumference were collected for titer detection by ELISA and were preserved at -80°C. Immunoglobulin was purified primarily from ascitic fluid using the ammonium sulfate precipitation technique. The concentration of monoclonal antibodies was determined by the BCA assay (Cowin Biotech, Beijing, China) (30).
3.7. Antibody Isotype Determination
Immunoglobulin isotypes were determined using the mouse monoclonal antibody isotyping kit (Southern Biotech, SBA clonotyping System-HRP), according to the manufacturer’s instructions.
4. Results
4.1. Plasmid Construction
Referring to the published sequence of monkey BV E2490 strains, the specific 951-bp fragment of gD corresponding to the extracellular region (amino acid sequence of 26 - 341) was synthesized by GENEWIZ Inc. (Suzhou, China) DNA synthesizer. The gene sequence was optimized based on the E. coli expression system codon preferences, while the amino acid sequence was maintained (Figure 1). The ideal percentage range of GC content of the gene was between 30% and 70%, after optimization; the average GC content was changed to 60% from the original 72%.
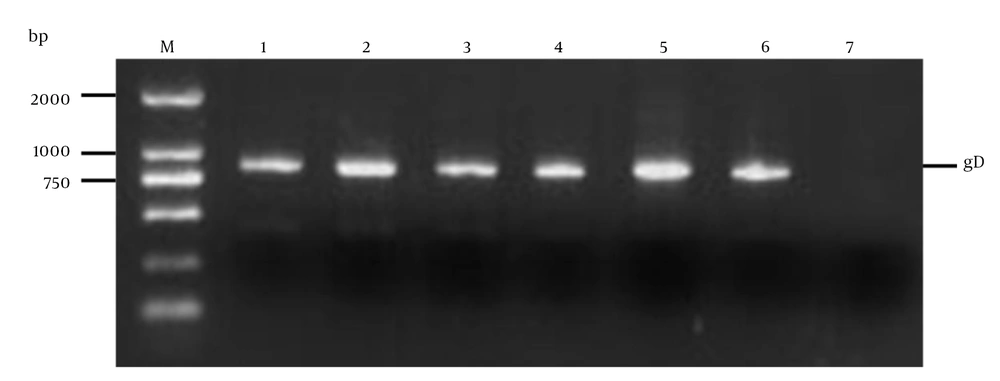
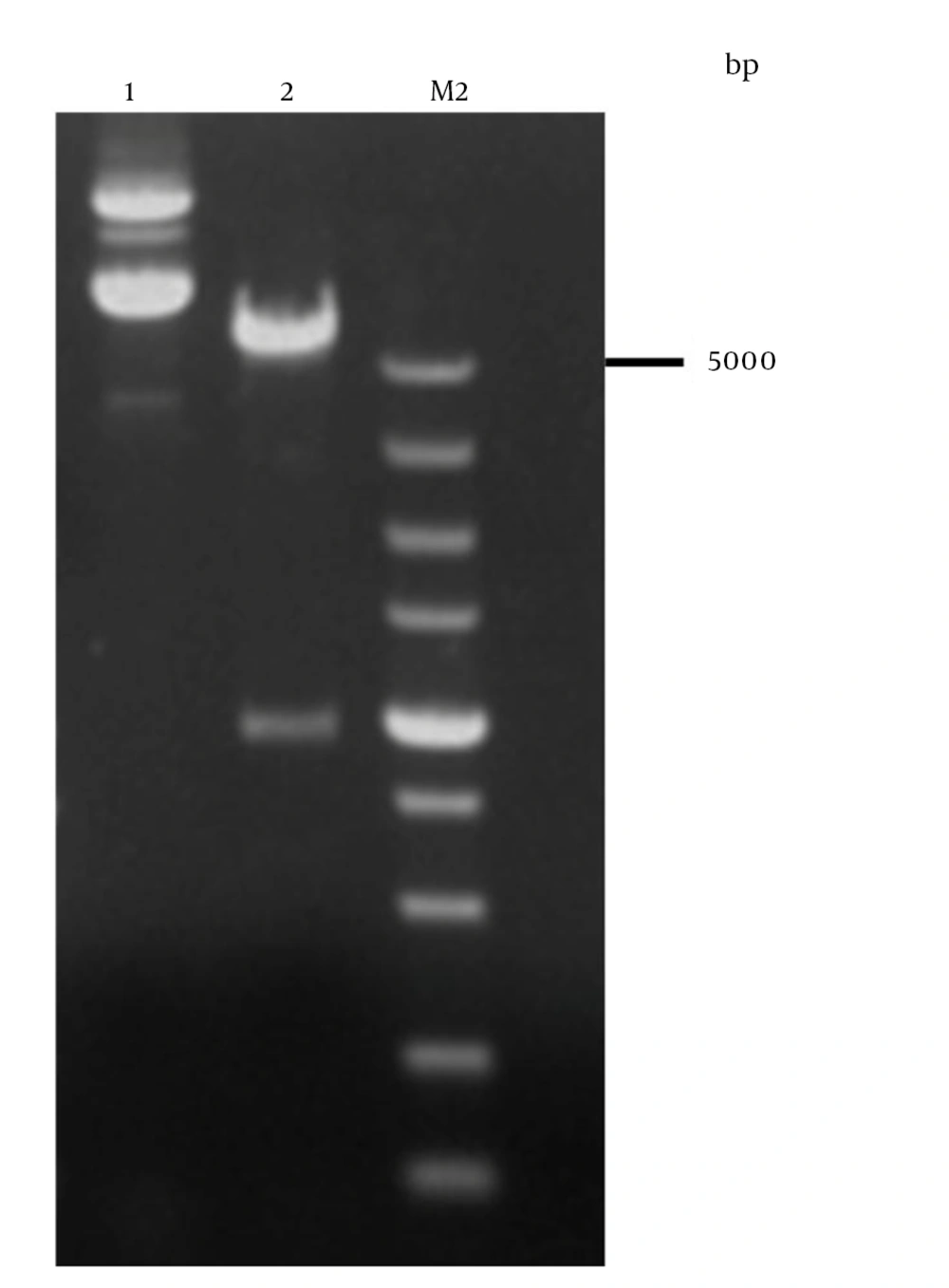
EcoR I and Hind III sites were designed in the primers to facilitate subsequent cloning. The vector of pET32a-gD was created by the gD gene, which was cloned in the prokaryotic expression vector pET-32a (+) (31). Inducible T7 RNA polymerase promoter was used to drive the expression of gD in this plasmid. The recombinant plasmid was verified by colony PCR (Figure 2), restriction enzymes digestion (Figure 3, lane 2) and analyzed by 1% agarose gel electrophoresis and sequenced (32). It was demonstrated that there was no mutation in the amino acid sequence by the sequencing results (data not shown).
4.2. Expression and Purification of the gD Protein
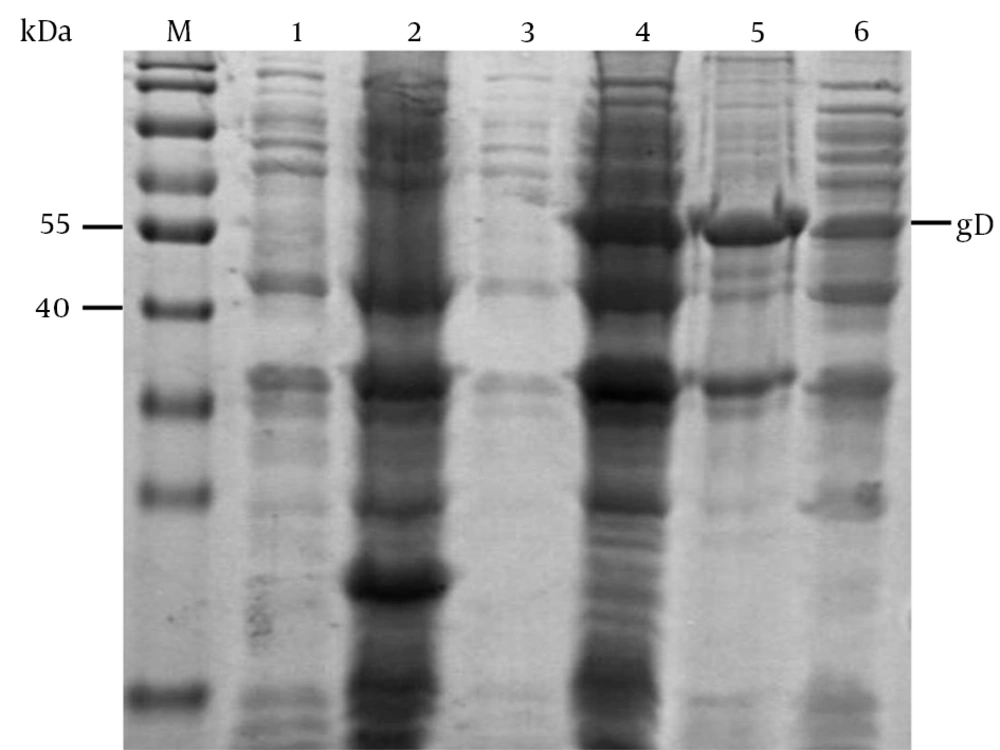
After cloning the gD gene into the prokaryotic expression vector pET-32a (+), the recombinant gD protein was successfully expressed at a high level in E. coli (33); its molecular weight was about 53 kDa. The protein was purified with Ni2+-NTA agarose resin, and was then eluted using various concentrations of imidazole for harvesting gD (Figure 5). The concentration of the purified His-tagged gD protein was 0.1 mg/mL, as shown by the results.
M, protein marker; 1, E. coli Rosetta (DE3) containing pET-32a (+) before induction; 2, E. coli Rosetta (DE3) containing pET-32a (+) was induced with IPTG; 3, E. coli Rosetta (DE3) containing pET-32a-gD before induction; 4, E. coli Rosetta (DE3) containing pET-32a-gD induced with IPTG; 5, inclusion bodies of the E. coli Rosetta (DE3) with pET-32a-gD induced by IPTG; 6, supernatants of the E. coli Rosetta (DE3) lysates with pET-32a-gD induced by IPTG.
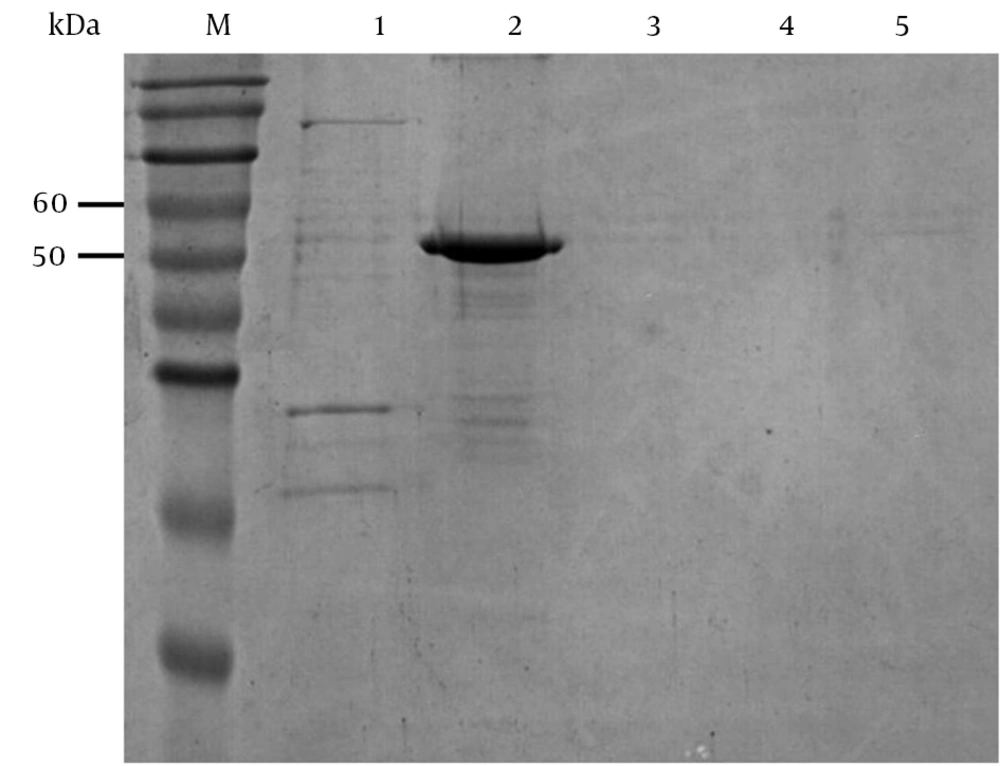
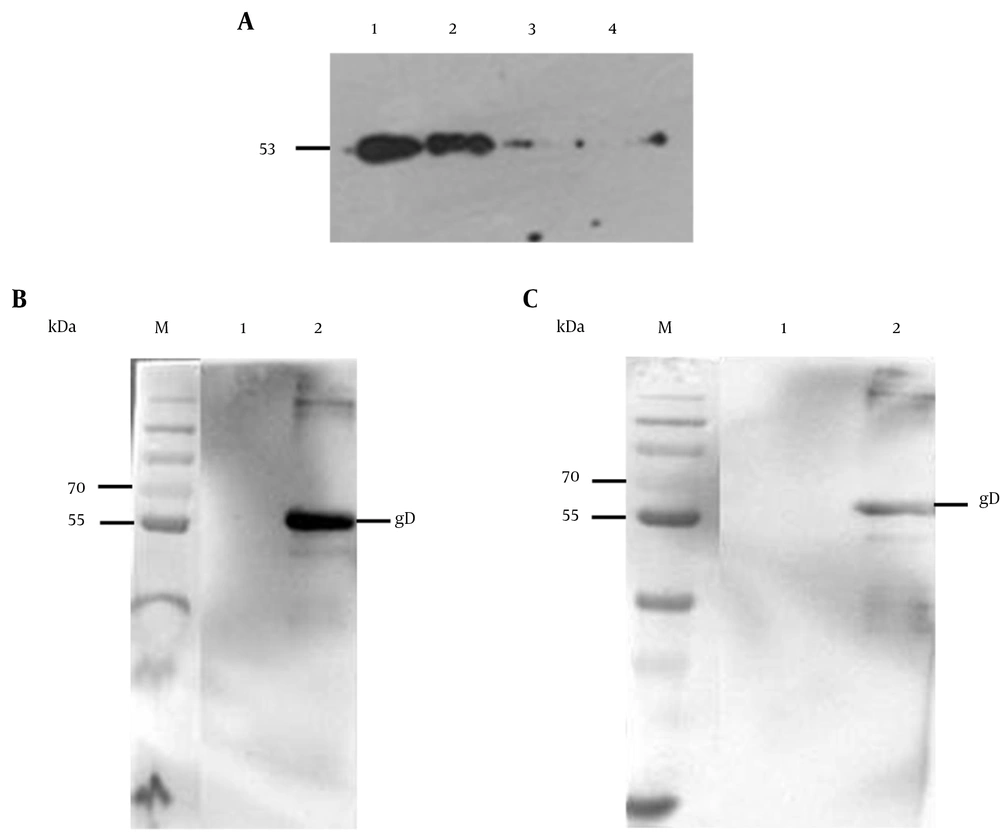
The purified protein was confirmed by western blot analysis with anti-His tag mAb and clinical BV positive monkey serum. The results showed that the recombinant gD protein revealed a specific band with expected molecular mass of 53 kDa, which was specifically recognized by anti-His monoclonal antibody (Figure 6A), and the picture of the electrophoresis also confirmed that the recombinant protein could specifically react with monkey B virus serum (Figure 6B). However, the negative serum of monkey had weak cross-reaction with the recombinant protein (Figure 6C). In the negative control, empty vector pET-32a (+) could not be induced to express the gD protein; it did not have a specific band in the western blot analysis (34).
A: 1, purified gD protein from E. coli Rosetta (DE3) containing pET-32a-gD; 2, IPTG induced E. coli Rosetta (DE3) containing pET-32a-gD; 3, non-induced E. coli Rosetta (DE3) containing pET-32a-gD; 4, IPTG induced E. coli Rosetta (DE3) containing pET-32a; B: M and 1, E. coli Rosetta (DE3) containing pET-32a-gD before induction; 2, purified E. coli Rosetta (DE3) containing pET-32a-gD; C: 1, E. coli Rosetta (DE3) containing pET-32a-gD before induction; 2, Purified E. coli Rosetta (DE3) containing pET-32a-gD.
4.3. Preparation of gD Protein-Specific mAb
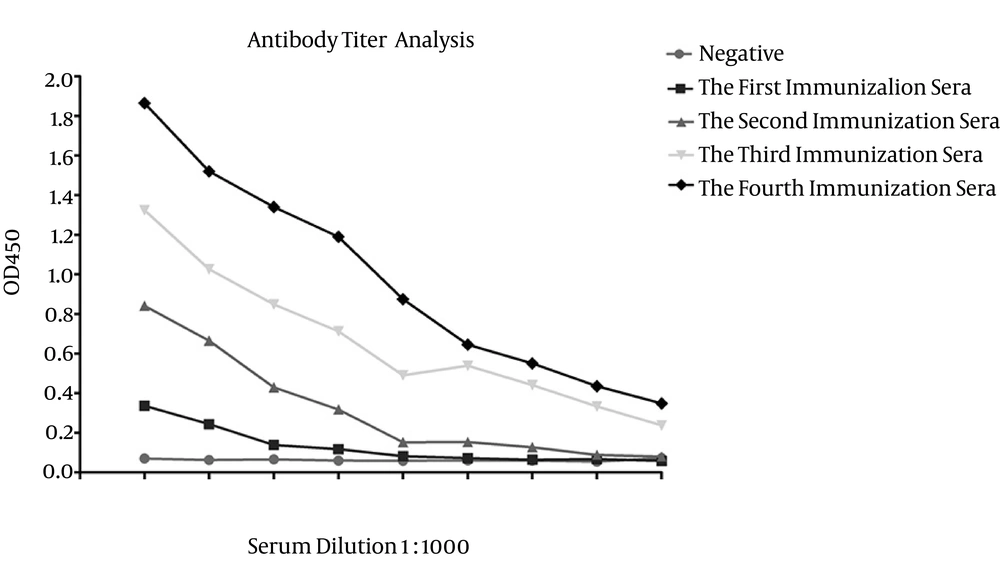
After the fourth immunization, ELISA was used to test the reactivity of sera, from immunized mice by the recombinant gD protein (Figure 7). In accordance with the above criteria, the mice could be used for mAbs production (35).
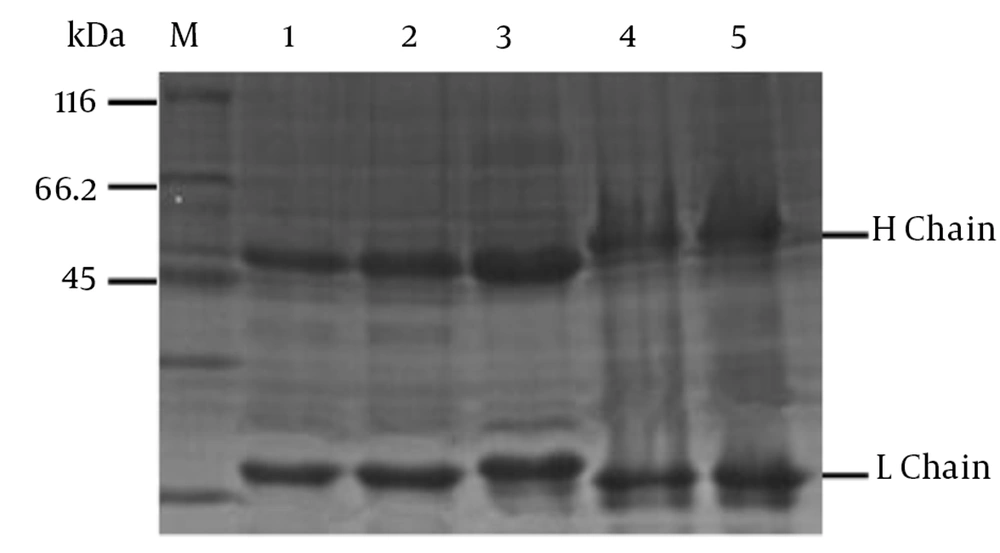
The culture supernatants of hybridoma cells from mAb were screened by indirect ELISA. Five stable positive hybridomas were cloned by three rounds of subclone and screening, designated as 4E3, 3F8, 3E7, 4B6 and 1H3, chosen to produce ascites (35). The immunoglobulin classes and subclasses of 4E3 and 1H3 were isotyped as IgG2b, while the other three mAbs (3F8, 4B6 and 3E7) were IgG1, and the light chains of all the five mAbs belonged to the kappa chain (Table 1) (36). The mAbs in ascites were purified and confirmed by SDS-PAGE (Figure 8). In accordance with the above criteria, the titers of those mAbs ranged from 10-2 - 10-6 (Table 1). The concentration of monoclonal antibodies is shown in Table 1. The mAb of 4E3 had the most sensitivity in the detection of B virus.
| MABs | Isotype | Ascites Titre | Ascites Concentration, mg/mL |
|---|---|---|---|
| 4E3 | IgG2b, κ chain | 10-6 | 3.5 |
| 3F8 | IgG1, κ chain | 10-5 | 3 |
| 3E7 | IgG1, κ chain | 10-5 | 2 |
| 1H3 | IgG2b, κ chain | 10-3 | 1.5 |
| 4B6 | IgG1, κ chain | 10-2 | 0.9 |
5. Discussion
In biomedical testing, the nonhuman primate research models are particularly valuable. For a wide variety of biomedical research endeavors the macaques are extremely valuable nonhuman primate models (e.g., a nonhuman primate model for AIDS) (3). The B virus, which is endemic to rhesus macaques, belongs to the family of herpesviridae. The fatality rate was 80% for infected individuals who were untreated (37). The macaque conventional populations were naturally infected by the B virus, which is a α-herpesvirus. A previous study showed the macaques, which carry numerous viruses will harm the researchers and impact the study results.
According to previous studies (38), for the diagnosis of many viral infections, recombinant DNA techniques generally play an important role. However, the use of recombinant antigens for BV sero-diagnosis has not been widely investigated, and it has been proposed that the specificity of BV gD is probably a valuable diagnostic reagent for distinguishing herpes B virus infections (23, 39, 40). The A + T and G + C content, rare codons, AT-rich stretches and mRNA secondary structure are factors that could distinctly affect the expression of foreign genes. According to the analysis of the coding sequence of gD, we found that it consists of GC-rich material. Therefore, we optimized the gene sequence to beneficial to the expression of gD. The results demonstrated that the anti-His mAb and BV positive monkey serum could recognize the purified gD protein specifically (Figure 6). MAbs is one of the valuable tools for specific epitopes and characterization of antigenic variation.
The splenocytes from the immunized mice were fused with SP2/0 myeloma cells. After three cycles of subcloning, five mAbs against the gD of B virus were obtained (41). After a test, we determined that our mAbs are stable by comparison with other studies of mAbs (42, 43). We will continue to track their stability. The SDS-PAGE analysis showed that five strains of mAb could have two distinct bands after purification. We used the monkey B virus serum instead of an infected person’s viral serum, which is because we did not have access to relevant serum of channels (8). However, we believe it is still possible to obtain reliable results. Sensitive reactions to recombinant antigen gD were shown by the monkey B virus serum and negative serum.
Although an inconsiderable cross-reaction was observed, the WB with the recombinant antigens was established to show specificity for the detection of B virus infections. Unfortunately, we only had HSV-2 virus and without HSV-1 virus and the standard strain of BV E2490, and the five monoclonal antibodies we screened had specificity and no cross-reactivity with HSV-2 by immunofluorescence assay (Data not shown). Whether they recognize HSV-1 needs further identification. Our research prepared abundant recombinant gD protein antigen, and this could be regarded as significant reference for the BV detection kit. The prepared mAbs could provide basis and a powerful tool for further research of the characteristics and function of envelope protein and its antibody.