1. Background
Acute Respiratory Infection (ARI) is the major cause of morbidity and mortality worldwide, especially in young children. Respiratory viruses such as Respiratory syncytial virus (RSV), influenza virus type A and B, Parainfluenza virus 1-3 and adenovirus are the most common pathogens affecting the respiratory tract of young children (1).
Human Metapneumovirus (hMPV) is associated with both upper and lower respiratory tract infections among infants and children worldwide (2). It was first identified as a novel respiratory virus in 2001 among children with ARI in the Netherlands. The hMPV belongs to the Paramyxoviridae family, Pneumovirinae subfamily, and the genus Metapneumovirus (3, 4).
The RSV is the most common etiological agent of viral lower respiratory tract infection and is considered as the most important cause of viral bronchiolitis in children. Respiratory syncytial virus belongs to the Paramyxoviridae family, Pneumovirinae subfamily, and is placed under the genus Pneumovirus (5, 6). The clinical presentation of hMPV infection ranges from a mild upper respiratory tract infection to severe lower respiratory tract disease accompanied by bronchiolitis and pneumonia (6). While RSV, Influenza virus and Parainfluenza virus are considered to be the most common respiratory viruses, the role of other less known viruses such as hMPV in ARI children remains largely unknown (7). Despite studies showing the prevalence of hMPV infection, the actual role of hMPV in children with severe ARI in Iran is poorly understood. Molecular techniques such as the polymerase chain reaction (PCR) are sensitive methods to define respiratory viruses that may be present in clinical specimens, especially in children suffering from respiratory infections (8).
2. Objectives
This study had two objectives. First, to determine the hMPV and RSV frequency and their co-infection in hospitalized ARI children, aged less than five years old, at one of the children’s referral hospitals in Iran. Second, to describe the seasonal prevalence of hMPV and RSV among this group from March 2010 till March 2013.
3. Patients and Methods
3.1. Patients and Samples
This descriptive study was based on the processing of nasopharyngeal or throat swabs from 158 children, aged under five years old, who were admitted to Aliasghar children hospital of Iran University of Medical Sciences with acute respiratory infections from March 2010 till March 2013; this was done for the determination of the frequency of hMPV and RSV infections and co-infections. The ARI children aged less than five years, having tachypnea with or without respiratory distress and cough, were included in this study. Children with ARI, aged above five years old, were excluded. The specimens were placed into a vial containing 1 mL of transport media and were sent to the Payvand clinical and specialty laboratory, where they were tested by real-time polymerase chain reaction for hMPV and RSV. The samples were divided into aliquots and stored at -80°C until use.
3.2. Nucleic Acid Extraction and cDNA Synthesis
Nucleic acids from specimens were extracted using the QIAamp viral RNA extraction mini kit (Qiagen GmbH, Hiden, Germany), according to the manufacturer’s recommended protocol. Reverse transcription was performed with the use of random hexamers and SuperScript first-strand (invitrogen), according to the manufacture’s instructions.
3.3. Real-Time Polymerase Chain Reaction
Real-time PCR was done using the Rotor-Gene 6000 from Corbet Research (Australia). The properties of the oligonucleotide primers and probes of hMPV and RSV are shown in Table 1 (9). The limit of detection for the hMPV and RSV real-time PCR are 100 copies per reaction. There are no cross-reaction between both viruses and other respiratory viruses such as influenza virus A and influenza virus B, parainfluenza virus 1 to 4, and adenovirus, implying a high specificity. Beta-2-microglobulin (B2-MG) DNA was used as the internal control to monitor nucleic acid extraction. The sequence of the primer and the probe of B2-MG are shown in Table 1 (10).
| Primer Or Probe Name | Primer Or Probe Sequence (5'-3') | Target Gene |
|---|---|---|
| hMPV-F | CAAGTGTGACATTGCTGAYCTRAA | F |
| hMPV-R | ACTGCCGCACAACATTTAGRAA | F |
| hMPV-P | FAM-TGGCYGTYAGCTTCAGTCAATTCAACAGA | F |
| RSV-F | AACAGATGTAAGCAGCTCCGTTATC | F |
| RSV-R | CGATTTTTATTGGATGCTGTACATTT | F |
| RSV-P | FAM-TGCCATAGCATGACACAATGGCTCCT | F |
| B2-MG-F | TGAGTATGCCTGCCGTGTGA | B2-MG |
| B2-MG-R | TGATGCTGCTTACATGTCTCGAT | B2-MG |
| B2-MG-P | FAM-CCATGTGACTTTGTCACAGCCCAAGATAGTT | B2-MG |
Abbreviations: B2-MG, beta-2-microglobulin; F, fusion.
The PCR conditions were as follows: reverse transcription at 50°C for 30 minutes, initial PCR denaturation at 95°C for 10 minutes, followed by 45 cycles of denaturation at 95°C for 15 seconds, annealing at 58°C for 60 seconds.
The positive controls comprised of cDNA of the viruses, provided by professor Dr. Eric C. J. Claas (Leiden university medical center, the Netherlands.).
The cycle threshold values of ≤ 39.9 were regarded as positive and cycle threshold values of ≥ 40 were regarded as negative. The results were discarded for any specimen with a negative internal control.
3.4. Statistical Analysis
Statistical analysis for comparison of relative values was performed by the z-test, using the STATA software version 8. P < 0.05 were considered statistically significant.
4. Results
4.1. Study Population
From March 2010 to March 2013, 158 specimens were obtained from hospitalized children under five years old for evaluation of RSV and hMPV infections. The majority of respiratory specimens (more than 65%) were from children less than one year old.
4.2. Respiratory Syncytial Virus and Human Metapneumovirus Prevalence
Overall, 31.1% (49/158) of samples were positive for RSV, and 5.7% (9/158) of the specimens were positive for hMPV. Among the 158 patient specimens, 63.2% (100/158) were negative for RSV and hMPV (Table 2). Respiratory syncytial virus and hMPV co-infection was not observed among them.
| Age Groups | Specimens Tested | Specimens Positive | RSV | hMPV |
|---|---|---|---|---|
| < 6 months | 78 (49.4) | 30 (19) | 29 (18.4) | 1 (0.6) |
| 6 - 12 months | 26 (16.4) | 8 (5.1) | 6 (3.8) | 2 (1.3) |
| 1 - 2 years | 20 (12.7) | 10 (6.3) | 8 (5.1) | 2 (1.3) |
| 2 - 5 years | 34 (21.5) | 10 (6.3) | 6 (3.8) | 4 (2.5) |
| Total | 158 (100) | 58 (36.7) | 49 (31.1) | 9 (5.7) |
aValues are expressed as No. (%)
4.3. Age and Gender Distribution of Respiratory Syncytial Virus and Human Metapneumovirus infections
Twenty-seven (55.2%) of the RSV-infected patients were females and 22 (44.8%) were males. Five (55.5%) of the hMPV-infected children were male while four (44.5%) were female. There were no significant differences between males and females. All patients with positive results for RSV and hMPV were divided into four age groups: < 6 months, 6 - 12 months, 1 - 2 years and 2 - 5 years. In the < 6 months group, the proportion of RSV cases was significantly greater (P < 0.05) than the proportion of hMPV cases, while in the children with 6 - 12 months, 1 - 2 years and 2 - 5 years, the proportions of RSV and hMPV were nearly the same (Table 2).
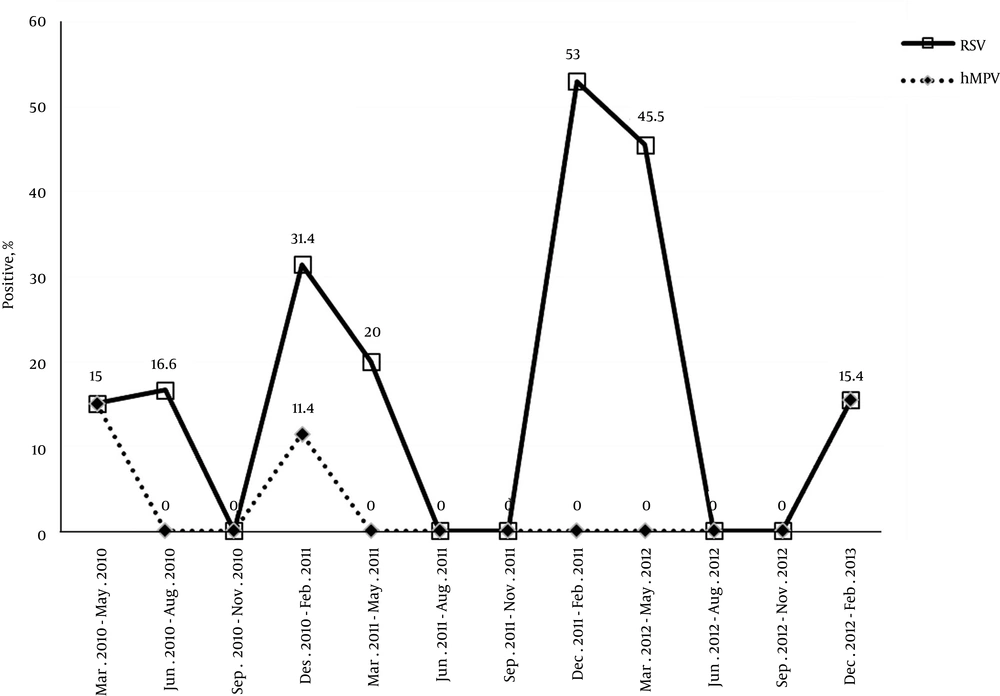
4.4. Seasonal Prevalence of Respiratory Syncytial Virus and Human Metapneumovirus Infections
The seasonal distribution of detected viruses over the three-year study period is shown in Figure 1, during which two peaks of RSV infections occurred annually, first during December 2010 to February 2011 (31.4%) and the second during December 2011 to February 2012 (53%). The hMPV infections were mainly detected from December 2010 to February 2011 and December 2012 to February 2013, when 11.4% and 15.4% of infections occurred, respectively.
5. Discussion
The study shows that 31.1% and 5.7% of RSV and hMPV infections, respectively, occurred among Iranian children < 5 years of age, hospitalized with acute respiratory tract infections. The prevalence of hMPV infection in Iranian children with ARI varied from 0.49% to 54.4%, which was not previously reported in Iran (11, 12). Herein, the proportion of acute respiratory infection of hMPV-positive cases among children under the age of 5 in our study (5.7%) was similar to studies from the Middle-East regions such as Amman (6%) and other regions such as Brazil (5.6%) (7, 13).
There are also other reports on the frequency of RSV in hospitalized children in Iran. Pourakbari et al. (14) studied hospitalized children under five years old with acute respiratory tract infection and found a prevalence of 17.2% for RSV, and Malekshahi et al. (11) assessed RSV among children less than six years old with ARI and reported that it was the cause of 16.8% cases (11, 14). In another study Nikfar et al. (15) observed that among 100 children with acute respiratory infection, 9% were infected with RSV. The above-mentioned results were lower than what was found in this study (31.1%), which is more similar to the findings of Moattari et al. (16) on hospitalized children with acute respiratory infection in south of Iran. They found that among 280 children under five years old, 84 (30.0%) were infected with RSV. There are several studies showing co-infection with RSV and hMPV (3, 5). Moattari et al. (16) observed that 10 (3.5%) of the studied patients were infected with both viruses, yet in the current study there was no co-infection with RSV and hMPV, which was similar to the findings of Mullins et al.(17). The difference between the prevalence rate of hMPV and RSV infections and co-infection with both viruses in several studies may be described by different groups of patients, methods used for detection of viruses, yearly variation in incidence and other variables, such as age, population density, socioeconomic factor and climate changes.
It was also found that hMPV-infected children were older than RSV-infected cases. The RSV was found predominantly in children < 1 year old, while hMPV infection occurred mainly in children aged 1 - 5 years. Such finding is similar to other studies on hMPV epidemiology (17-20). The reason of such difference may be explained by longer lasting maternal immunity to hMPV compared with RSV.
The hMPV seasonal distribution was determined by the peaks within the winter and the spring. This finding was observed in other hMPV studies as well (8, 21). In addition, RSV infection occurred largely during the winter months, similar to those previously observed elsewhere(5, 7, 22, 23). In accordance with several studies, the seasonal occurrence of hMPV infection mainly overlaps with RSV infections (8, 24). Hence it seems that hMPV is less important than RSV as a cause of ARI, especially in children under one year old and the seasonal occurrence of both viruses is the same. Large-scale follow-up epidemiological studies are needed to fully explore all respiratory viruses causing ARI in Iranian young children and assess seasonally patterns.