1. Background
Despite attempts to understand and control Mycobacterium tuberculosis for more than a century, this pathogen remains a pressing public health threat, infecting more than one-third of the world’s population and causing almost two million deaths yearly (1). Understanding the molecular mechanism of the host/pathogen interaction is believed to be vital for controlling M. tuberculosis infections.
Previous research showed that vaccines constructed based on ESAT6 and CFP10 could reduce the M. tuberculosis burden in guinea pigs and non-human primates (2, 3). However, substantial evidence indicates that ESAT6 and CFP10 are also virulence factors. The Rv3875 gene that encodes ESAT6 is located within the region of difference 1 (RD1), which is present in all pathogenic mycobacteria, including M. tuberculosis and M. bovis, but not in attenuated M. bovis bacillus Calmette-Gue’rin (BCG) (4). ESAT6 and CFP10 are secreted as 1:1 dimers through a specific secretion system, ESX-1 (5-7). Studies of M. bovis BCG and RD1-complemented M. bovis BCG have demonstrated that the secretion of ESAT6 and CFP10 are required for the virulence, pathogenicity, and growth of M. bovis in macrophages and in mice (8, 9). In addition, ESAT6 and CFP10 are produced in the lungs of infected mice (10). The mechanisms by which ESAT6 and CFP10 contribute to virulence are not fully defined, but are believed to be involved in the cytolysis of alveolar epithelial cells and macrophages (11), favoring the intercellular spread of M. tuberculosis (12).
Nitric oxide (NO) is formed when the guanidine nitrogen of L-arginine is oxidized by a family of isoenzymes known as NO synthases. The high expression of NO in response to cytokines or to pathogen-derived molecules is important for host defense against intracellular microorganisms, such as Toxoplasma gondii, Listeria monocytogenes, Ectromelia virus, and M. tuberculosis (13-15). Recent studies showed that exposure of M. tuberculosis to NO at low concentrations killed more than 99% of the bacteria in culture (16), indicating that NO may induce macrophage apoptosis. In a TB-infection murine model, NO played an essential role in the mononuclear phagocyte killing of M. tuberculosis (17).
2. Objectives
Considering that understanding the interactions between M. tuberculosis and macrophages may be propitious for controlling tuberculosis (TB), we discussed the different effects of the recombinant ESAT6-CFP10 protein on macrophages with the host’s first and second exposures to M. tuberculosis.
3. Materials and Methods
3.1. Expression and Purification of Recombinant ESAT6-CFP10 Protein
The recombinant fusion ESAT6-CFP10 gene was generated using overlap PCR. First, the CFP10 and ESAT6 genes were amplified from the genome of M. bovis strain C68201 (provided by Dr. Ding Jiabo, China institute of veterinary drug control) with two pairs of gene-specific primers (Table 1 (P1 and P2, P3 and P4, respectively)), which was synthesized by BGI Tech (China). The two resultant PCR products were gel-purified, then used as templates for the fusion gene ESAT6-CFP10, which was created with overlap PCR using the gene-specific primers P1 and P4. The PCR amplified ESAT6-CFP10 gene product was then cloned into the BamHI and HindIII sites of the pET-32a(+) expression vector. The recombinant plasmid was transformed into Escherichia coli BL21 (DE3) host cells for histidine (His)-tagged ESAT6-CFP10 protein expression.
| Primer | Sequence (5’- 3’) | Restriction Sites |
|---|---|---|
| P1 | ccGGATCCatggcagagatgaagac | BamH I |
| P2 | GCTGCCGCCACCGCCGCTTCCGCCACCGCCGCTTCCACCGCCACCgaagcccatttgcgaggacagcgcct | - |
| P3 | GGTGGCGGTGGAAGCGGCGGTGGCGGAAGCGGCGGTGGCGGCAGCatgacagagcagcagtggaatttcgcgg | - |
| P4 | ccAAGCTTtgcgaacatcccagtga | Hind III |
| P5 | ccAAGCTTgccaccatggcagagatgaagac | Hind III |
| P6 | ccGGATCCcgtgcgaacatcccagtga | BamH I |
Primer Sequences for ESAT6-CFP10 PCR Amplification
The expression of the His-tagged ESAT6-CFP10 protein was induced by the addition of isopropyl β-D-l-thiogalactopyranoside (IPTG). The expression of the His-ESAT6-CFP10 protein was detected by SDS-PAGE with Coomassie Blue staining. The recombinant fusion protein was purified using a His-bind purification kit (Qiagen, Germany) according to the manufacturer’s instructions. The contaminating endotoxin (lipopolysaccharide) was removed from the purified recombinant protein using a ProteoSpinTM Endotoxin Removal Micro kit (Norgen Biotek Corporation, Canada) following the standard protocols provided by the manufacturer. The purified proteins were quantified with the Enhanced BCA Protein Assay Kit (Beyotime, China).
3.2. Western Blotting
The His-ESAT6-CFP10 protein was verified by Western blotting. In brief, the purified protein was electrophoresed on 12% SDS-PAGE, then electrotransferred to nitrocellulose membranes. The membranes were incubated at room temperature for 2 hours in PBST containing 5% skimmed milk to block non-specific membrane binding. This was followed by probing the membranes with bovine serum raised against M. bovis at room temperature for 2 hours. The membranes were then washed 3 times with PBST and subsequently incubated at room temperature for 2 hours with horse radish peroxidase (HRP)-conjugated goat anti-bovine IgG (Sigma, USA) as the secondary antibody. The membranes were then washed again in PBST and signals were detected using super signal west pico chemiluminescent substrate (Pierce, USA), according to the manufacturer’s instructions.
3.3. Construction of Recombinant Eukaryotic Expression Vector pEGFP-N1-ESAT6-CFP10
The ESAT6-CFP10 gene was amplified with gene-specific primers (Table 1 (P5 and P6)) synthesized by BGI Tech (China), then cloned into the BamHI and HindIII sites of the pEGFP-N1 expression vector to generate the recombinant eukaryotic plasmid designated as pEGFP-N1-ESAT6-CFP10, containing a green fluorescent protein (GFP). The plasmid was confirmed by restriction analysis and gene sequencing.
3.4. Generation of the Rat Alveolar Macrophage Cell Line Expressing ESAT6-CFP10 Protein
NR8383 rat alveolar macrophages (purchased from Life and science research institute of Shanghai) were used in these experiments. The NR8383 cells were cultured in complete RPMI-1640 medium (Hyclone, USA) supplemented with 10% fetal bovine serum (Gibco, USA) and 100 μg/mL of penicillin and streptomycin. In order to obtain NR8383 cells expressing ESAT6-CFP10, the NR8383 cells were transfected with the recombinant eukaryotic plasmid pEGFP-N1-ESAT6-CFP10. One day before transfection, 2.0 × 105/mL cells were seeded onto 24-well plates and cultured at 37°C in a humidified incubator with 5% CO2. The transfections were performed with Lipofectamine® 2000 reagent (Invitrogen, USA), according to the manufacturer’s instructions. Subsequently, the transfected cells were cultured with complete RPMI-1640 medium for 18 hours. The transfected cells were selected using 10 μg/mL of G418 (Gibco, USA) until a stable NR8383 cell line expressing ESAT6-CFP10 was obtained. In addition, to further confirm and characterize the stable cell line, green fluorescence signals were detected with a fluorescence microscope (Nikon, Japan). The stable NR8383 cell line expressing ESAT6-CFP10 protein was named NR8383-EC.
3.5. Detection of ESAT6-CFP10 mRNA in NR8383-EC Cells by RT-PCR
The total RNA was extracted from the cells using TRIzol® Reagent (Invitrogen, USA). To avoid contamination with genomic DNA, the total RNA samples were treated with RNase-Free DNase I (Takara, Japan). First-strand cDNA was synthesized using M-MLV reverse transcriptase (Invitrogen, USA) according to the manufacturer’s instructions. Using the cDNA as a template, the ESAT6-CFP10 mRNA was amplified by PCR with gene-specific primers P1 and P4 (Table 1). The PCR products were run on a 1.0% (w/v) agarose gel and detected with ethidium bromide under UV light.
3.6. Cell Proliferation Assay
The proliferation of NR8383 or NR8383-EC in response to recombinant protein His-ESAT6-CFP10 was determined with a WST-1 assay using a WST-1 Cell proliferation and cytotoxicity assay kit (Beyotime, China). In brief, the NR8383 or NR8383-EC cells were seeded onto 96-well plates at a density of 2 × 104 cells/well and stimulated with recombinant protein His-ESAT6-CFP10 at concentrations of 10 μg/mL. At the same time, the purified product from the BL21 (DE3) (pET-32a) vector was used as a control. The cells were incubated at 37°C with 5% CO2 for 24 hours, followed by incubation with 10 μL of WST-1 for 1 hour. The absorbance was determined at 450 nm using an automatic microplate reader (Biotech, USA). The relative proliferation rate was calculated as ODtreated cells/ODuntreated cells × 100%. The experiments were conducted in triplicate, at least 3 times.
3.7. Measurement of NO Synthesis
The effect of recombinant protein His-ESAT6-CFP10 on NO generation by NR8383 and NR8383-EC cells was determined with the NO assay kit (Beyotime, China) according to the manufacturer's instructions. Briefly, the NR8383 and NR8383-EC cells were seeded onto 96-well plates at a density of 2 × 104 cells/well, and pre-treated with or without LPS (1 μg/mL) for 4 hours. The cells were then stimulated with or without purified recombinant protein His-ESAT6-CFP10 (10 μg/mL) for 24 hours. Subsequently, Griess reagent I and Griess reagent II were added into the cell supernatants, and the optical density was measured at 540 nm using an automatic microplate reader. Sodium nitrite (NaNO2) was used to generate a standard cure. The experiments were conducted in triplicate, at least 3 times.
3.8. Statistical Analysis
All results were presented as mean ± standard deviation (SD) from at least three independent experiments. Statistical analyses were performed with a two-tailed Student’s t test. Significant differences were assigned P values of < 0.05, < 0.01, and < 0.001, which were denoted by *, **, and ***, respectively. All statistical values were calculated with SPSS 19.0 software (IBM, USA).
4. Results
4.1. Expression and Purification of Recombinant His-ESAT6-CFP10 Protein
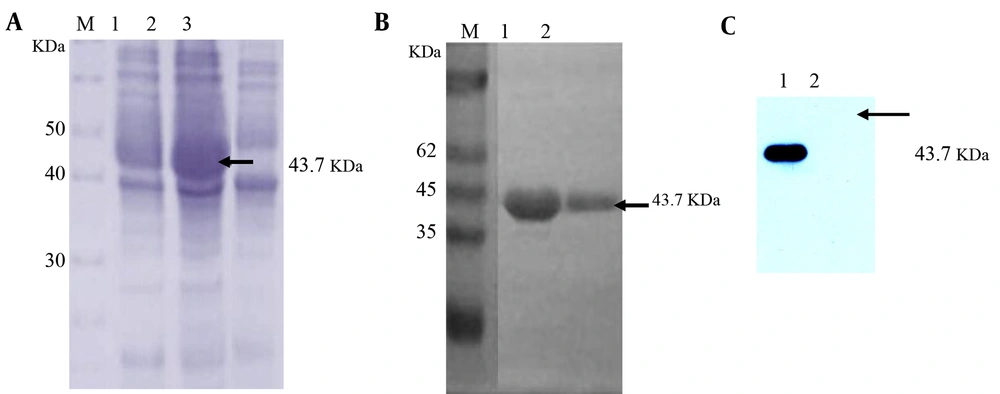
The ESAT6-CFP10 fusion gene was generated from the genome of M. bovis strain c68201 by overlap PCR amplification. It was then inserted into a pET-32a(+) expression vector, thereby generating a recombinant expression plasmid pET-32a-ESAT6-CFP10. The recombinant expression plasmid vector was confirmed by gene sequencing. The recombinant plasmid was transformed into E. coli BL21 (DE3) for His-ESAT6-CFP10 production. Expression of the His-ESAT6-CFP10 protein was induced with the addition of 1 mM IPTG. The expressed proteins were analyzed with SDS-PAGE and Western blotting (Figure 1). A Coomassie Blue-stained protein band was observed migrating with a molecular weight of about 43.7 KDa, which was consistent with the expected molecular weight (Figure 1A), indicating that the ESAT6-CFP10 gene was successfully expressed in E. coli BL21(DE3). Western blotting analysis with bovine positive serum raised against M. bovis further validated the expression of the recombinant His-ESAT6-CFP10 fusion protein (Figure 1C). The His-ESAT6-CFP10 protein was purified, and SDS-PAGE showed that a high-purity recombinant His-ESAT6-CFP10 was obtained (Figure 1B).
A, the SDS-PAGE analysis of the recombinant protein. M, middle-range protein marker; lane 1, BL21(DE3)(pET-32a-ESAT6-CFP10) in the absence of IPTG; lane 2, BL21(DE3)(pET-32a-ESAT6-CFP10) induced by IPTG; lane 3, BL21(DE3)(pET-32a) induced by IPTG. B, the purified His-ESAT6-CFP10 protein analyzed by SDS-PAGE. M, unstained protein molecular weight marker; lanes 1 - 2, purified His-ESAT6-CFP10 protein; C, the Western blotting analysis of recombinant protein using bovine positive serum raised against M. bovis; lane 1, purified recombinant fusion His-ESAT6-CFP10 protein; lane 2, His protein. Arrows indicate the positions of the target proteins.
4.2. Generation of Rat Alveolar Macrophage Cell Line NR8383-EC Expressing ESAT6-CFP10 Protein
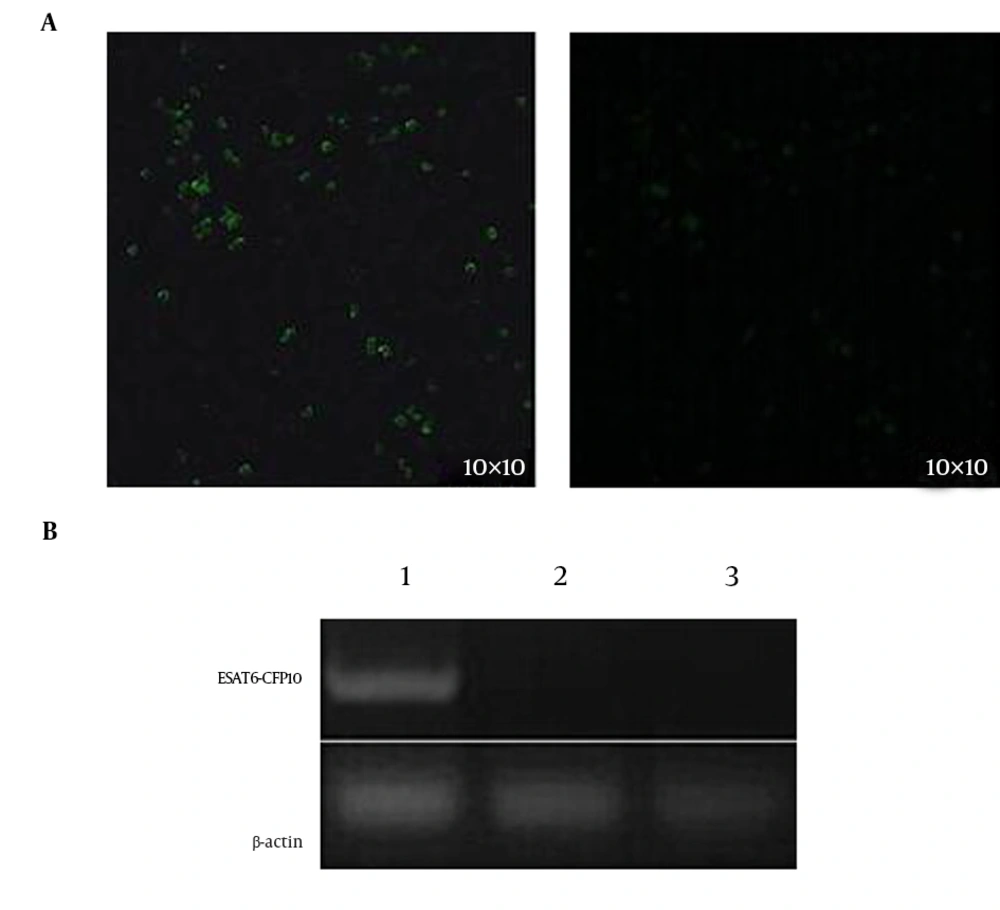
The recombinant eukaryotic expression plasmid pEGFP-N1-ESAT6-CFP10 was constructed, then confirmed by restriction analysis and gene sequencing. The ESAT6-CFP10 gene was fused to the N terminal of GFP in the recombinant plasmid. For generating the NR8383 rat alveolar macrophage cell line expressing the ESAT6-CFP10 protein, the NR8383 cells were transfected with pEGFP-N1-ESAT6-CFP10 using Lipofectamine 2000, and were screened with 10 μg/mL of G418. The green fluorescence signals were detected in NR8383-EC rat alveolar macrophages transfected with recombinant pEGFP-N1-ESAT6-CFP10 plasmid, but not in the non-transfected NR8383 macrophages (Figure 2A), which indirectly showed the expression of the ESAT6-CFP10 fusion protein in the NR8383-EC cells. The expression of ESAT6-CFP10 mRNA in NR8383-EC cells was analyzed by RT-PCR. PCR products of predicted sizes for ESAT6-CFP10 (630 bp) were obtained from the NR8383-EC cells (Figure 2B).
The ESAT6-CFP10 was fused to the N terminal of GFP of the pEGFP-N1 expression vector. A, the expression of the fusion protein was detected with fluorescence signals, using a fluorescence microscope; 1, NR8383 transfected with the recombinant eukaryotic plasmid pEGFP-N1-ESAT6-CFP10; 2, NR8383 non-transfected with the recombinant eukaryotic plasmid pEGFP-N1-ESAT6-CFP10; B, the mRNA level of ESAT6-CFP10 was analyzed with RT-PCR using total RNA extracted from cells; lane 1, the gene amplified from the NR8383-EC cells; lane 2, the gene amplified from NR8383 cells transfected with pEGFP-N1 plasmid; Lane 3, the gene amplified from non-transfected NR8383 rat alveolar macrophages.
4.3. Cell Proliferation and NO Production Capacity Assays
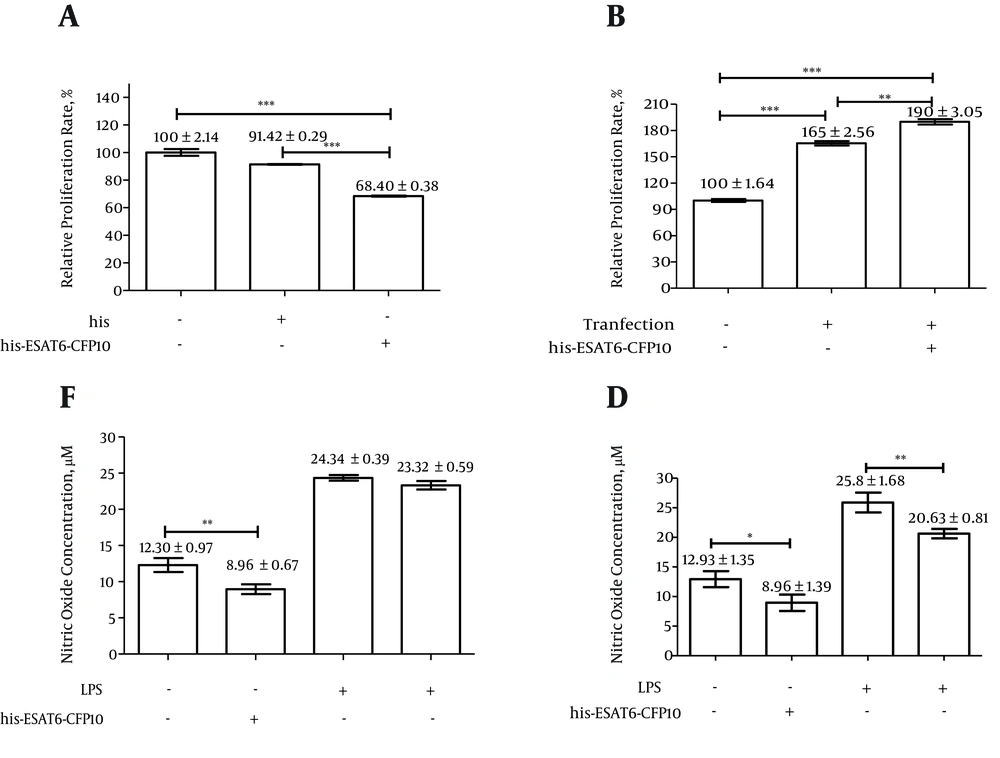
The NR8383-EC cell line and NR8383 rat alveolar macrophages were stimulated using His-ESAT6-CFP10 protein and His protein, respectively. WST-1 analysis and Griess reactions showed that recombinant protein His-ESAT6-CFP10 affected the cell proliferation and NO generation, respectively. The ESAT6-CFP10 protein inhibited the proliferation of NR8383 rat alveolar macrophages (Figure 3A), but promoted the proliferation of NR8383-EC cells (Figure 3B). However, it also inhibited the NO generation of both NR8383 rat alveolar macrophages (Figure 3C) and NR8383-EC cells (Figure 3D).
A, the proliferation of NR8383 or B, NR8383-EC in response to recombinant protein His-ESAT6-CFP10 was determined by WST-1 assay. The cells were stimulated with recombinant protein His-ESAT6-CFP10 at concentrations of 10 μg/mL, then proliferation was determined. At the same time, the purified product from BL21(DE3) (pET-32a) was used as a control. The NO generation effect of recombinant protein His-ESAT6-CFP10 on C, NR8383 or D, NR8383-EC was determined by the NO assay kit, according to the manufacturer’s instructions. The cells were pre-treated with or without LPS (1 μg/mL) for 4 hours, and were then stimulated with or without purified recombinant protein His-ESAT6-CFP10 (10 μg/mL) for 24 hours. NO generation was then determined. *P < 0.05, **P < 0.01, ***P < 0.001. The experiment was repeated three times.
5. Discussion
Mycobacterium tuberculosis is the primary causative agent of human tuberculosis, which remains a major global health problem. M. tuberculosis is a typical intracellular pathogen that resides within the host macrophages and dendritic cells. Invasion of macrophages by M. tuberculosis is a critical step in the establishment of tuberculosis (TB) infections. Macrophage apoptosis is a host defense mechanism against M. tuberculosis infection (18, 19). On the other hand, M. tuberculosis could survive in the host macrophages by inhibiting their apoptosis. Some researchers have indicated that avirulent mycobacterial species, such as the attenuated M. bovis BCG, cause considerably higher rates of macrophage apoptosis than do the virulent M. tuberculosis species (20). Comparative analyses of the genomic sequences of M. tuberculosis (21) and M. bovis (22) with that of the attenuated M. bovis BCG strain have identified a number of proteins deleted in BCG. These include the small secreted ESAT6 and CFP10, which are known to contribute to microbial virulence. Therefore, the effects of ESAT6 and CFP10 on macrophage apoptosis are very important for studying and understanding the mechanisms of tuberculosis.
In this study, the His-ESAT6-CFP10 fusion protein was expressed in an E. coli system, which was confirmed by SDS-PAGE and Western blot analysis. The SDS-PAGE analysis confirmed the successful production of the fusion protein in soluble form. This form was thought to be the best condition for the test, because of its complete natural structure. The Western blot analysis confirmed that the immunogenicity of the fusion protein was in good condition. These results also indicated that the fusion expression of ESAT6 and CFP10 has no negative effect on the biological activity of these key proteins involved in tuberculosis virulence.
Obtaining a cell line that expresses the ESAT6-CFP10 fusion protein is very important for the study of the interactions between these proteins and macrophages during M. tuberculosis infections. In this study, using a eukaryotic expression plasmid pEGFP-N1-ESAT6-CFP10, a NR8383-EC rat alveolar macrophage cell line that expresses ESAT6-CFP10 protein was constructed. It was verified by RT-PCR and fluorescence assay. The ESAT6-CFP10 gene was amplified from the NR8383-EC cDNA by RT-PCR, and fluorescence was detected in the NR8383-EC cells. Hence, these combined results showed that the NR8383-EC cell line expressing the ESAT6-CFP10 protein was constructed successfully.
To understand the effects of M. tuberculosis on macrophages when the host is challenged with M. tuberculosis the first and second times, the His-ESAT6-CFP10 protein was used to stimulate the response of the NR8383 rat alveolar macrophages and the NR8383-EC macrophage cell line that expressed ESAT6-CFP10 protein. The effects of recombinant His-ESAT6-CFP10 protein on NR8383 and NR8383-EC were then determined by measuring the cell proliferation and NO generation.
The results of NO generation showed that the recombinant His-ESAT6-CFP10 protein inhibited the NO generation of both the NR8383 rat alveolar macrophages and the NR8383-EC cell line. However, the cell proliferation results showed that the recombinant protein His-ESAT6-CFP10 inhibited the proliferation of NR8383 rat alveolar macrophages, but promoted the proliferation of the NR8383-EC cell line. Therefore, these results indicated that M. tuberculosis could escape the host immune defense by continuous inhibition of NO generation, but it could promote or inhibit the apoptosis of macrophages in order to regulate the number of cells by some responses or interactions (23) when the host is first infected by M. tuberculosis or when it again comes into contact M. tuberculosis. This was evidenced by differences in proliferation between the NR8383-EC cell line, which carried the ESAT6-CFP10 fusion protein, and NR8383, which did not. These findings lead us to believe that significantly different responses protect the host from M. tuberculosis infections between the first and second M. tuberculosis exposures.
In conclusion, the NR8383-EC cell line and recombinant His-ESAT6-CFP10 protein were successfully constructed. The His-ESAT6-CFP10 protein stimulation of the NR8383-EC cell line and of the NR8383 rat alveolar macrophages showed different effects. These results provide novel valuable information and new ideas for studying the mechanisms of infection and the diagnostic methods for tuberculosis.