1. Background
Hepatitis C virus (HCV) is a major health issue around the world, which has been described as a cause of chronic hepatitis with potential liver failure, liver cirrhosis and hepatocellular carcinoma (1). Today, infected people are more than 170 million around the world (2). Many attempts have been made to achieve new drugs or vaccines for control and therapy. Moreover, there is no protective vaccine and effective therapeutic options for major genotypes of hepatitis C virus. Therefore, there is an immediate need for the development of an efficacious HCV vaccine (3).
Successful clearance of the acute form of disease is associated with strong HCV-specific T cell responses, while chronic and persistent infection is characterized by inefficient activities in T cells, therefore, an immunotherapeutic vaccine approach that stimulates cellular immunity against HCV antigens could be efficient in chronic HCV disease (4). DNA vaccines by the advantages of being cost efficient, easily produced and safe are an effective approach to induce immune responses against structural and non-structural (NS) HCV genes in mice (5, 6).
Non-structural proteins such as non-Structural protein 3 (NS3) of HCV genome, because of induction of strong immunity and the existence of conserved regions, are attractive for vaccine development (7). The NS3 with proteinase, helicase and NTPase activity is a necessary factor during the process of replication and translation (4). Several findings have now reported that T-cell immune responses against NS3 correlate with resolution of the infection (8).
Numerous studies have indicated the immunosuppressive effects of full-length NS3 protein, due to enzymatic activity and induction of apoptosis in mature dendritic cells and cytotoxic lymphocytes. It seems that removing the enzymatic activity of NS3 and the selection of immunogenic fragment could be able to boost HCV specific immune response (9, 10).
2. Objectives
In the present study, a recombinant plasmid producing the HCV immunogenic fragment of NS3 protein (amino acid 1095 - 1379 of HCV genome), was evaluated in mice for its ability to induce immune responses.
3. Materials and Methods
3.1. RNA Extraction and cDNA Synthesis
Viral RNA was extracted from the plasma of an Iranian patient with HCV subtype 1a, according to the manual of the viral nucleic acid extraction kit (Roche, Germany). cDNA synthesis was performed on 10 μL of the extracted RNA with 4 μL mixture of reverse transcription (RT) buffer, 1 μL RNase inhibitor, 1 μL dNTP mixture (10 mM), 1 μL random hexamer primer, and 1 μL MMLV RT enzyme (Thermo Scientific, CA). The reaction was carried out at 42°C for 60 minutes and 72°C for 15 minutes in a final step for inactivation of RT enzyme.
3.2. Polymerase Chain Reaction Amplification and Cloning of Recombinant Plasmid
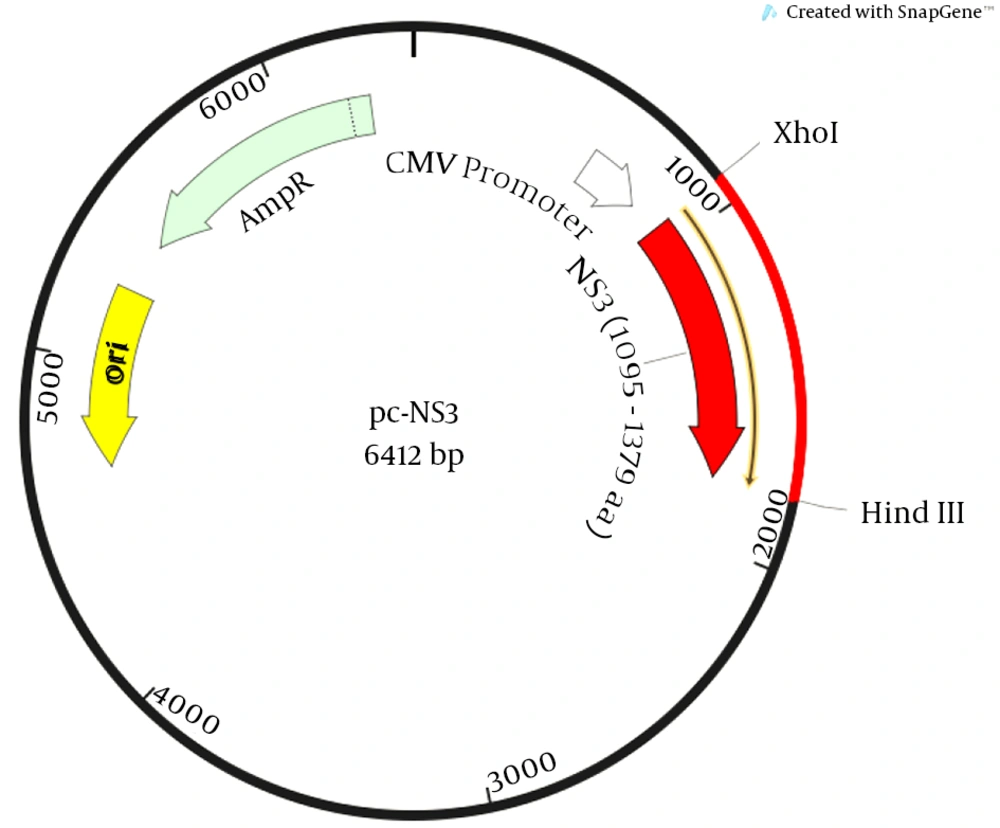
To amplify the NS3 fragment of HCV, PCR was performed on the synthesized cDNA. The immunogenic and conserved region was selected for designing the primers. Semi-nested PCR was carried out with forward and reverse 1 primers (Table 1) for first step, and forward and reverse 2 primers (Table 1) for the second step. The PCR reaction had a total volume of 25 μL, containing 1.25 unit (0.5 μL) Pfu DNA polymerase, 1 μL (10 pmol/μL) of each of the forward and reverse primers, 2.5 μL of 10X reaction buffer, 1 μL dNTPs (10 mM), 5 μL template, and water, which was added into the mixture. Polymerase chain reaction products of the first PCR were used as the template for the nested PCR. The PCR was performed according to the following program: initial denaturation at 94°C for seven minutes, 38 cycles including denaturation at 94°C for 45 seconds, annealing at 58°C in the first PCR and 59°C in the second PCR for 30 seconds, extension at 72°C for 95 seconds, and final extension at 72°C for five minutes. After electrophoresis using 1.5% agarose gel containing safe stain DNA, the PCR product was visualized under a UV transilluminator and purified with the PCR product purification kit (Roche, Germany). The purified PCR product and pCDNA3.1 was digested with XhoI and HindIII restriction enzyme and then, ligation was performed according to the thermo protocol kit (Thermo Scientific, CA). The ligated product (pc-NS3, Figure 1) was transformed into Escherichia coli DH5α strain and these bacteria were subsequently cultured in LB agar plate containing 50 μg/mL of ampicillin. For cloning confirmation, restriction enzyme analysis and bidirectional sequencing were performed.
| Primer Name | Primer Sequence |
|---|---|
| Forward primer | 5' TAC TCG AGC ATG GCT CCT GTC ATC CAG ATG TAT AC 3' |
| Reverse primer 1 | 5' ATG ATG GTG GTG GAT AGC CTT GCC ATA GAA G 3' |
| Reverse primer 2 | 5' GCG AAG CTT ATT AAT GAT GAT GAT GGT GGT GG 3' |
3.3. Expression of Recombinant NS3 Fragment
Expression of NS3 fragment was analyzed in transfected 293T cells with the pc-NS3 by the turbofect kit (Thermo Scientific, CA). One day after transfection, the medium was changed with fresh medium to grow for 48 hours. Finally, the transfected cells were collected to detect the NS3 proteins. The un-transfected cell served as the negative control. The collected cells were washed twice in phosphate buffered saline (PBS) and treated with lysis buffer (1% Nonidet P-40, 10 mg/L- phenyl methylsulfonyl fluoride 50 mM-tris Cl, pH 8.0) for 30 minutes on ice and then the lysates were centrifuged at 800 g for 15 minutes. The supernatants were collected to be analyzed by the western-blot method.
3.4. Western Blotting
The fragment of the NS3 proteins, were resolved by 15% sodium dodecyl sulfate (SDS) gel. The protein bands were transferred to nitrocellulose membranes (Amersham, United Kingdom). Subsequently, the membrane was blocked with blocking buffer (3% non-fat skimmed plus 0.05% tween 20) for 1.5 hours at room temperature; then, the membrane was incubated with 1:1000 diluted anti-beta actin antibody (Abcam, UK) or monoclonal NS3 antibody (monoclonal anti-hepatitis C virus NS3 antibody against 1340 to 1470 amino acids of HCV polyproteins, Abcam, UK) in blocking buffer as the primary antibody for two hours at room temperature, then the membrane was washed three times for five minutes with TBST (tris buffered saline plus 0.1% Tween 20). After washing, the membrane was incubated with horseradish peroxidase (HRP)-conjugated anti goat against IgG (Invitrogen, Germany), diluted at 1:5000 in TBST for one hour at RT and then, washing of the membrane was performed as mentioned above. chemiluminescent HRP substrate (Amersham, United Kingdom) was added to the membrane for seven minutes at room temperature. Finally, the produced light was recorded on a film.
3.5. Protocol of Immunization
Six to eight week-old female BALB/c (H-2d) mice, were obtained from the animal house of the Pasteur institute of Iran. All experiments were approved by the local animal ethics council (ethics number: D52/639) and were performed in accordance with the national experimental guidelines. Groups of seven mice were immunized at weeks 0, 2, 4 and 6 intradermally close to the base of the mice tail with 100 µg of purified plasmids (pc-NS3 and pcDNA3.1). Mouse blood samples were collected at weeks five and eight by retro-orbital bleeding and sacrificed for further investigation.
3.6. Evaluation of Humoral Immune Responses
Blood was collected at weeks 0, 5 and 8 after immunization from all groups with a capillary tube via retro-orbital puncture. All sera were kept in a -70°C freezer before total and subtype antibody assay. The antibody response against the conserved NS3 fragment was evaluated by the enzyme-linked immunosorbent assay (ELISA) with recombinant antigens. Furthermore, 3 µg/mL of recombinant NS3 fragment expressed in E. coli, were coated in a volume of 100 µL in 96 well plates and incubated overnight at 4°C. The plates were then blocked by incubation with PBS buffer containing 3% bovine serum albumin (BSA) at 37°C for one hour. After three times washing with PBS/Tween, 100 µL of immunized mice serum (200 × diluted) was added to the wells followed by one hour of incubation at 37°C. Humoral antibodies were detected with HRP-conjugated goat anti-mouse IgG antibody (dilution 1:10000 at 37°C, Sigma, UK) followed by addition of 3, 3’, 5, 5’-tetramethylbenzidine (TMB).The reaction was stopped by addition of 0.5 N H2SO4, and the OD450 nm was measured using an ELISA plate reader. The IgG titer was determined by end-point dilution. IgG antibody subclasses were measured as described above by goat anti-mouse IgG1, IgG2a, IgG2b and IgG3 antibodies (dilution 1:2000 at room temperature, Sigma, UK) and HRP-labeled anti-goat IgG conjugate (dilution 1:10000 at room temperature).
3.7. Cell Proliferation Assays
Two weeks after the last injection, five mice of each group were sacrificed according animal ethics protocols and spleens were removed aseptically. Proliferation assays were performed by using the Roche cell proliferation ELISA, BrdU (5-bromo-2 deoxyuridine, Roche, Germany). Briefly, spleen cells were isolated by Roswell park memorial institute (RPMI) 1640 injection in spleens. Red blood cells (RBCs) were lysed with 0.1 M ammonium chloride. The splenocytes were then washed two times with PBS and then suspended in complete RPMI 1640 (supplemented with 10% fetal bovine serum (Gibco, NY), 2 mM L-glutamine, 100 mM HEPES, 100 IU/mL of penicillin and 100 µg/mL of streptomycin (Gibco, NY)). The cells were counted after staining with trypan blue for the viability assay. The spleen cells were cultured in triplicates (1 × 106 cells/well) in a 24-well culture plate stimulated with 3 µg/mL of recombinant NS3, and incubated at 37°C under 5% CO2 and 95% humidity. For positive control Con A (concanavalin A, 5 μg/mL; Sigma, USA) was added and un-stimulated wells were used as negative controls. After 60 hours, 20 µL/well BrdU solution was added and re-incubated for an additional 12 hours at 37°C, and then the proliferation analysis was performed according to the colorimetric BrdU assay protocol (Roche, Germany). Stimulation index was obtained from (OD stimulated blank)/(OD un-stimulated blank).
3.8. ELISPOT Cytokines Assay
The frequency of IL-4 and IFN-γ secreting cells was determined using ELISPOT kits (Mabtech, Sweden), according to the manufacturer’s manual. Briefly, 96-well plates were separately coated overnight at 4°C with anti-IL-4 monoclonal capture antibody. The IL-4 coated and pre-coated IFN-γ plates were washed and blocked with PBS and skimmed milk, respectively. Splenocytes from immunized and control mice (1 × 105 cells/well) were cultured with recombinant NS3 protein for 40 hours at 5% CO2 and 37°C to allow production and capture of the released cytokines. After incubation, cells removal and washing, corresponding plates were incubated with biotin-conjugated anti-IFN-γ and anti-IL-4 monoclonal antibodies. Subsequent incubation with streptavidin-alkaline phosphatase and BCIP-NBT substrate led to the appearance of spot-forming cells (SFCs) that were counted under a dissection stereoscope (Leica microscopy system, Heerbrugg, Switzerland). Wells containing Con A (5 µg/mL) and irrelevant peptide (132 - 145 residues from HCV core antigen; DLMGYIPLVGPLG) served as positive and negative controls, respectively.
3.9. Statistical Analysis
Statistical analysis was carried out with Student’s t-test and one-way analysis of variance (ANOVA) to evaluate the differences of the changes among all experiments. Values of P < 0.05 were considered significant.
4. Results
4.1. Polymerase Chain Reaction Amplification and Construction of Recombinant Plasmid
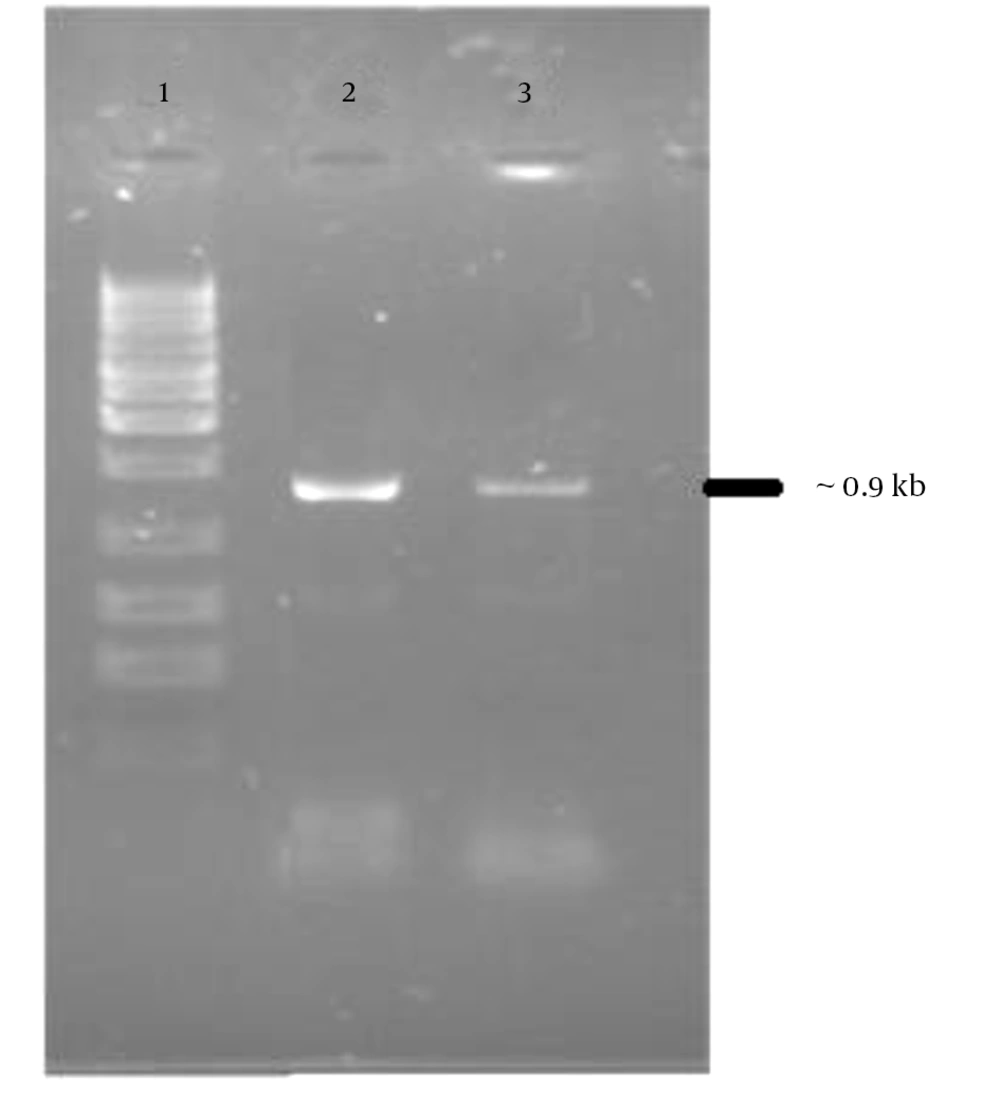
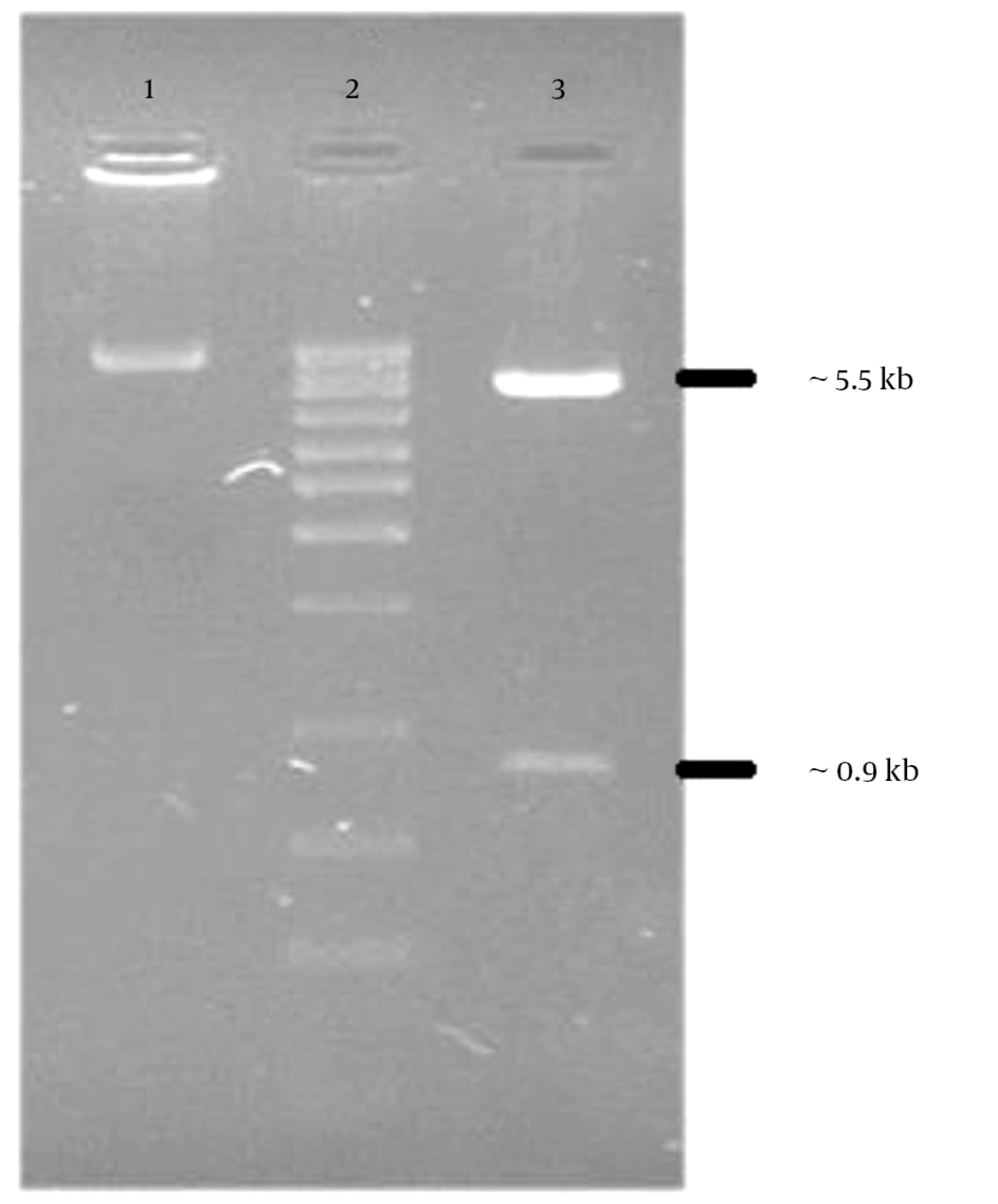
The PCR products were analyzed on 1.5% agarose gel in parallel with 1 kb DNA ladder marker (Figure 1). The expected 902 bp PCR product fragment was observed (Figure 1). The PCR product was ligated to pcDNA3.1 expression vector and transformed into the E. coli DH5α strain. The recombinant plasmid (Figure 2) was confirmed by restriction enzyme analysis (Figure 3) and sequencing analysis.
The recombinant plasmid (pc-NS3) was digested with Xho I and Hind III. The digested plasmid were separated on 1.5% agarose gel and visualized after ethidium bromide staining. Lane 1, undigested pc-NS3; lane2, 1kb DNA ladder marker; lane 3, pc-NS3 digested yields 5.5 kb and 0.9 kb restriction fragments.
4.2. Expression of the NS3 Proteins in Mammalian Cells
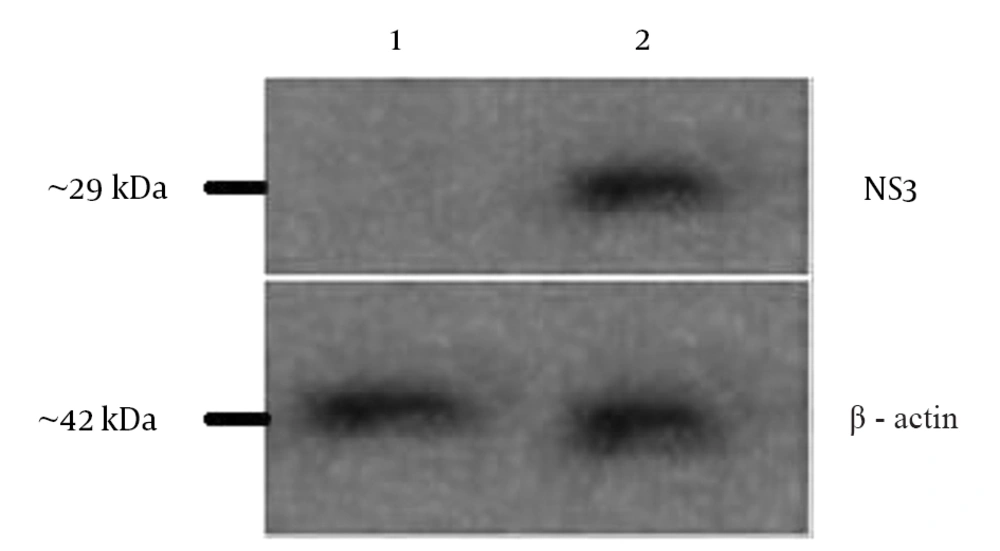
The expression of HCV NS3 gene was analyzed in transfected HEK293 T cells by western blotting using anti-hepatitis C NS3 antibody (Abcam, UK). Non-transfected HEK293 T cell lysate was used as the negative control. The western blot result revealed expression of NS3 fragment in transfected HEK293 T lysate. There was no expression in the cells transfected with pcDNA3.1 and non-transfected (negative control) HEK293 T cells (Figure 4).
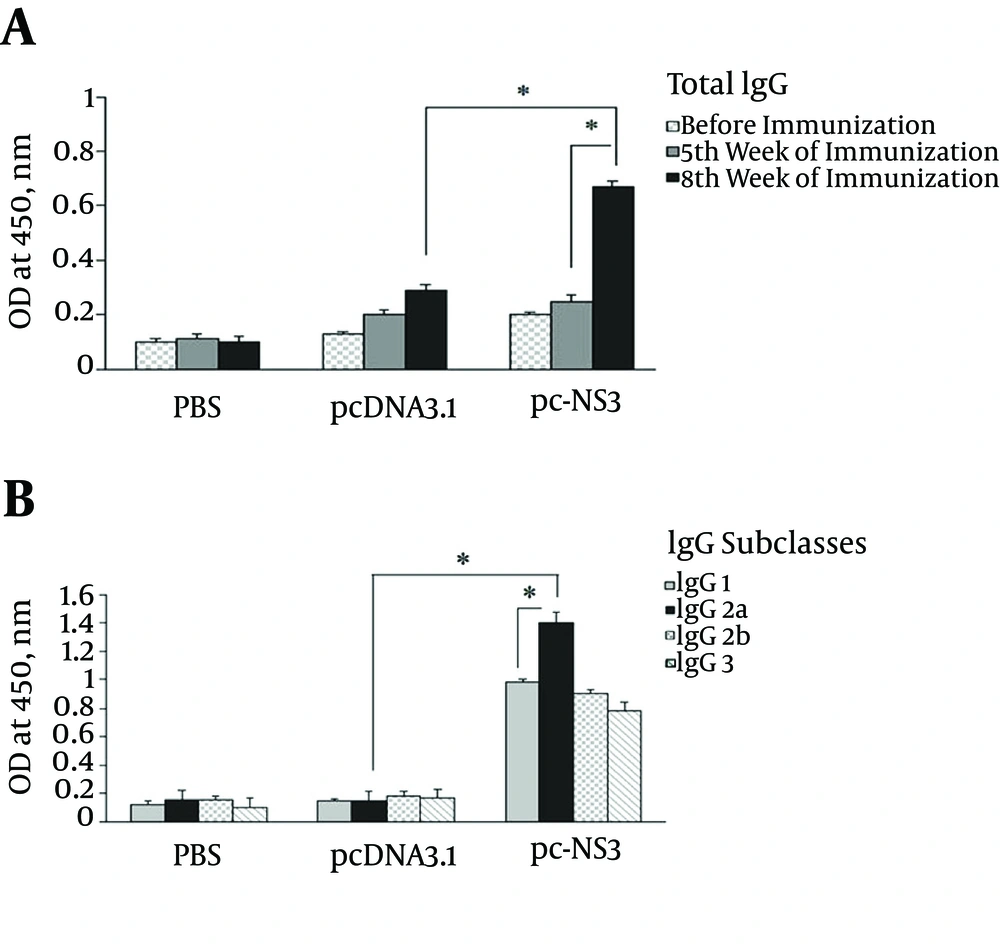
4.3. Effect of Immunization Regimen on Humoral Response
As shown in Figure 5, the animal group vaccinated with pcDNA3.1-NS3 induced NS3-specific total IgG. Accordingly, the recombinant plasmid was capable of inducing higher levels of total IgG, in comparison with the negative control group. It was also interesting to see that evaluation of IgG isotypes indicated that IgG2a was the predominant isotype that was respectively followed by IgG2b. This suggests that the immune responses induced by pc-NS3 injection favour the Th1 pathway. Of note, sera of the negative control groups (either injected with PBS) did not show any specific reactivity to the NS3 fragment protein, while almost all the test groups demonstrated IgG antibody response within two weeks after the forth injection that was dramatically increased after the forth immunization.
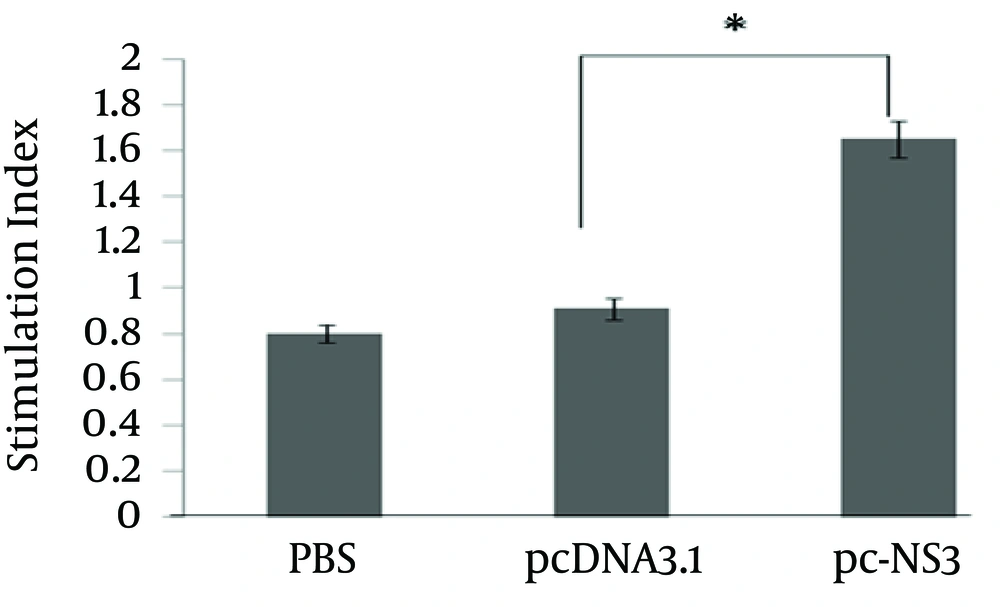
4.4. Cell Proliferation Assay
To analyze lymphocyte proliferation, splenocytes of immunized mice were stimulated with NS3 protein while incorporation of BrdU into the stimulated splenocytes was detected by ELISA. The results indicated that the mice immunized with pc-NS3 had a significantly higher SI mean compared with PBS or pcDNA3.1 (P < 0.05) (Figure 6).
Splenocytes of immunized and control mice were cultured and pulsed with NS3 peptide. The proliferative response was measured by cell proliferation ELISA, BrdU colorimetric kit (Roche Diagnostics, Germany) as per the manufacturer’s protocol. The data represents mean SI of two determinations ± S.D. (* indicates statistical significance, P value < 0.05).
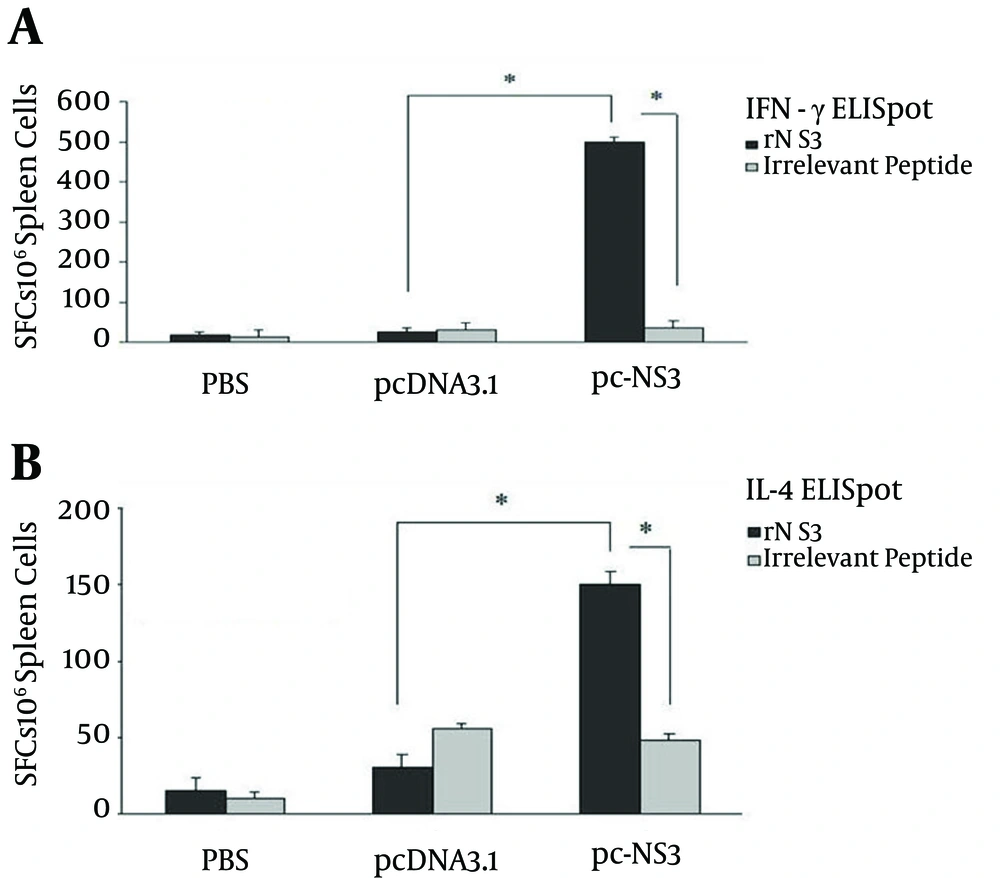
4.5. Effect of Immunization Regimen on Cellular Response
As shown in Figure 7, the assay of cytokine profile was performed by IFN-γ and IL4 ELISpot. In contrast to the control groups (pcDNa3.1 and PBS) splenocytes of mice vaccinated with recombinant plasmid efficiently induced both specific IFN-γ and IL4-producing cells. However, the vaccinated mice promoted significantly higher numbers of IFN-γ-secreting cells in comparison with the control groups (P < 0.05). This data confirmed the potency of recombinant NS3 plasmid immunization regimen and the induction of a potent cellular immunity.
5. Discussion
Currently, there is no vaccine against HCV (3); high rate of mutagenicity and diversity in the HCV genome may be a major obstacle for vaccine design (11). Previous findings indicate that cell-mediated immune response may be necessary for an effective vaccine (12). Promising vaccine candidates, such as DNA vaccines, have been shown to have many advantages for inducing strong T cell responses (13). Unlike immunization with recombinant proteins, which shift to produce Th2 responses, DNA vaccines are able to elicit strong Th1 responses due to the ability of antigens to be processed intracellularly (13). In addition, DNA vaccines theoretically have unlimited boosting capability and can be re-administered as often as desired without fear of inducing antibodies against the plasmid, as is the case with recombinant viral vectors.
In previous studies the use of structural HCV proteins have shown limited humoral immunity response that is specific for homologous virus isolate and this strategy was not sufficient to achieve virus clearance (14). Therefore, due to stability and conserved feature of HCV, a non-structural protein is a great target for vaccine design. The importance of NS3 in viral replication and being conserved between different strains, make it an attractive candidate for T cell based vaccines (15). Due to the importance of NS3 specific T cell responses in resolution of acute infection and control of chronic infection, as well as the clear advantages of DNA immunization. It seems that NS3 could be a great target for designing a DNA vaccine. On the other hand, currently there are a few studies, which indicate the immunosuppressive effects of full length NS3 protein (8, 9). It seems that a new vaccine based on removing the protease activity of NS3 and the selection of immunogenic fragment, would be able to boost immune response (15).
With the aim to develop an effective DNA HCV vaccine, we designed and administered an HCV NS3 DNA vaccine and then performed humoral response, cell proliferation and cytokine assays. The results demonstrated that the construct, pc-NS3, is expressed in cell culture and immunization with it resulted in T cell proliferation and a strong immune response in mice, which is important for antiviral effects. The same group also demonstrated higher levels of IFN-γ and IL-4 secretion compared to the control groups.
The humoral immunity assay indicated that pc-NS3 can induce significant high level total IgG, where frequency of immunizations can affect the magnitude of this response. Our data is in accordance with previous studies done by Grubor-Bauk et al. (16) and Yu et al. (17) in which NS3 DNA vaccine and dendritic cells (DCs) containing HCV NS3 protein induced high interferon-gamma production, enhanced cytotoxicity and strong lymphocyte proliferation.
Mikkelsen et al. showed that recombinant adenoviruses expressing the truncated form of NS3 elicit robust specific humoral and cellular immunity (18). In another study, Encke et al. showed that HCV NS genes are effective vaccine targets that induce cellular immunity in immunized mice and can protect tumor formation in a tumor mice model (19). Accordingly, NS genes, including NS3, can induce cellular immunity. Gao et al. showed that pHCV-NS3-Th1 (fusion of CD4+ T cell epitope peptide of NS3 and class II associated invariant chain peptide (CLIP) segment) resulted in high cellular proliferation and IFN-γ production. In pHCV-NS3-Th1-treated groups, CD4+ proliferation was significantly higher, yet the pHCV-NS3 group failed to produce suitable IL-4 cytokine (20).
Lazdina et al. showed that after two NS3/NS4A gene immunizations, high specific antibody titers were detected in mice, dominated by IgG2a and IgG2b, however DNA-based immunizations were much less potent than recombinant NS3 protein in humoral response, although some factors, specially, the priming of a more Th1-like CD4+ T cell population by DNA vaccination may be desired in therapeutic vaccination (21).
The results demonstrated that the immunogenic truncated region of NS3 could be used to develop DNA vaccines against hepatitis C. However more studies are needed in this regard in the future.