1. Background
Ralstonia mannitolilytica is an emerging opportunistic pathogen that is widely present in external environments, such as water, soils, and plant surfaces (1). Ralstonia mannitolilytica rarely causes clinical infections, but once it does, it can lead to more serious infections, such as sepsis, meningitis, and osteomyelitis. There have been pediatric outbreaks of R. mannitolilytica in the United States due oxygen transport equipment contamination (1). Hospital outbreaks of Ralstonia spp. are mainly associated with the contamination of treatment water or auxiliary treatment instruments (1-5). There have been several reported cases of systemic infections caused by R. mannitolilytica (6, 7), mostly occurring in immunocompromised patients. Clinical isolation of R. mannitolilytica is rare in China, while separation from the blood is even rarer (8).
Ralstonia mannitolilytica-caused septicemia is a relatively rare clinical diagnosis, and its drug resistance is serious. Three cases of bloodstream infections with R. mannitolilytica occurred at our hospital between October and November 2013, all of which developed after gastric cancer surgery or a hepatic hemangioma. There is no typical clinical manifestation of R. mannitolilytica infection, and the lack of sufficient experience with its diagnosis and treatment limits doctors’ understanding of this disease.
2. Objectives
In this report, we summarize the clinical infection characteristics of 3 cases, and analyze their risk factors. We also report the drug susceptibility testing and nosocomial infection investigation process, hoping to contribute to the understanding of this infectious bacteria.
3. Patients and Methods
3.1. Patients
The retrospective review of these cases and the collection of their follow-up data were approved by the hospital ethics committee. All patients signed written informed consent forms.
The 3 cases of R. mannitolilytica-induced septicemia in our hospital all occurred after tumor surgery. All of these patients developed postoperative fever with a maximum temperature of 39.8 °C during October and November 2013 at the 2nd Affiliated Hospital of Wenzhou Medical University. The patients were 1 man and 2 women, with an average age of 61.7 ± 8.7 years. During disease onset, all 3 of the patients had septic shock symptoms, including chills, fever, reduced or undetectable blood pressure, increased heart rate, and decreased urine output. When the disease became severe, the patients were immediately transferred to the intensive care unit (ICU). The laboratory tests indicated a progressive reduction in the numbers of white blood cells (WBCs) and platelets, and significant increases in C-reactive protein (CRP), procalcitonin (PCT), and endotoxins. R. mannitolilytica was detected in the blood cultures. The patients’ information, including underlying diseases, infection symptoms, antimicrobial drug use, catheterization, mechanical ventilation, laboratory detection indicators, and clinical outcomes, are shown in Table 1.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Gender | Female | Male | Female |
| Age, y | 74 | 56 | 55 |
| Diagnosis at admission | Gastric T-cell lymphoma | Gastric cardia carcinoma | Hepatic hemangioma |
| Underlying disease(s) | Diabetes mellitus, hypertension, and tuberculous pleurisy for 2 years | - | Diabetes mellitus and hypertension |
| Surgery | Distal gastrectomy | Radical resection of gastric cardia carcinoma | Partial resection of left hepatic lobe |
| Central venous catheterization, d | 10 | 11 | 6 |
| Mechanical ventilation, d | 9 | 5 | 5 |
| Clinical manifestation | Chills, persistent fever, increased heart rate, hypotension shock, indifference, decreased urine output | ||
| Temperature at disease onset, °C | 39.1 | 38 | 37.9 |
| WBC count, × 109/L | (1.6 - 4.39) | (18.2 - 2.58) | (0.6 - 3.6) |
| Platelet count, × 109/L | (254 - 59) | (130 - 51) | (73 - 22) |
| CRP, mg/L | 175.0 | 64.00 | 138.00 |
| Endotoxin, pg/mL | 33.47 | 28.35 | 76.16 |
| PCT, ng/ml | > 100.0 | 36.680 | 52.740 |
| Brain natriuretic peptide (BNP), pg/mL | 2.130 | - | 1.230 |
| Therapeutic drug | IPM1, (TZP2) | IPM, TZP | TZP |
| Outcome | Cured | Cured | Cured |
Summary of Clinical Information of 3 Patients With R. mannitolilytica-Induced Septicemia
3.2. Isolation, Culture, and Identification of Bacterial Strains
Blood was drawn from the patients under aseptic conditions, then 5 mL samples of the blood were injected into aerobic and anaerobic culture flasks and cultured with the blood culture instrument (BacT/ALERT® 3D 120, bioMerieux Inc., Marcy L’Etoile, France). The culture results were positive 24 hours later, and were transferred onto sheep blood agar plates, chocolate plates, and MacConkey agar plates (all purchased from Yilan Bio Co., Ltd. Hangzhou, China). Anaerobic gas bag cultivation was adopted and blood was cultured at 35°C; aerobic cultures were maintained in an incubator at 35°C containing 5% CO2. Bacterial identification was performed using the automated microbial identification system VITEK Compact-2 (bioMerieux Inc., Marcy L’Etoile, France).
3.3. Drug-Susceptibility Testing
The drug-susceptibility tests were performed using the mass spectrometry microbial identification system VITEK MS (bioMerieux Inc., Marcy L’Etoile, France) and the agar dilution method. The results were analyzed according to the instructions of the Clinical and laboratory standards institute (CLSI) (9).
3.4. Growth Experiments in Sterile Distilled Water
In order to simulate the actual living environment of environmental pathogenic bacteria, the growth experiment was performed in sterile distilled water. The isolated strains were prepared as bacterial suspensions at a turbidity of 0.5 McFarland standards (approximately 107 colony-forming units [CFU]/mL). A volume of 50 µL of suspension was sterilely removed to a clean bottle containing 5 mL of sterile distilled water, to make a 105 CFU/mL bacterial solution. Samples were then cultured at 37°C, room temperature, or 4°C. Each week, the samples were inoculated on blood agar plates to observe the bacterial growth.
3.5. Homology Analysis of Bacterial Strains
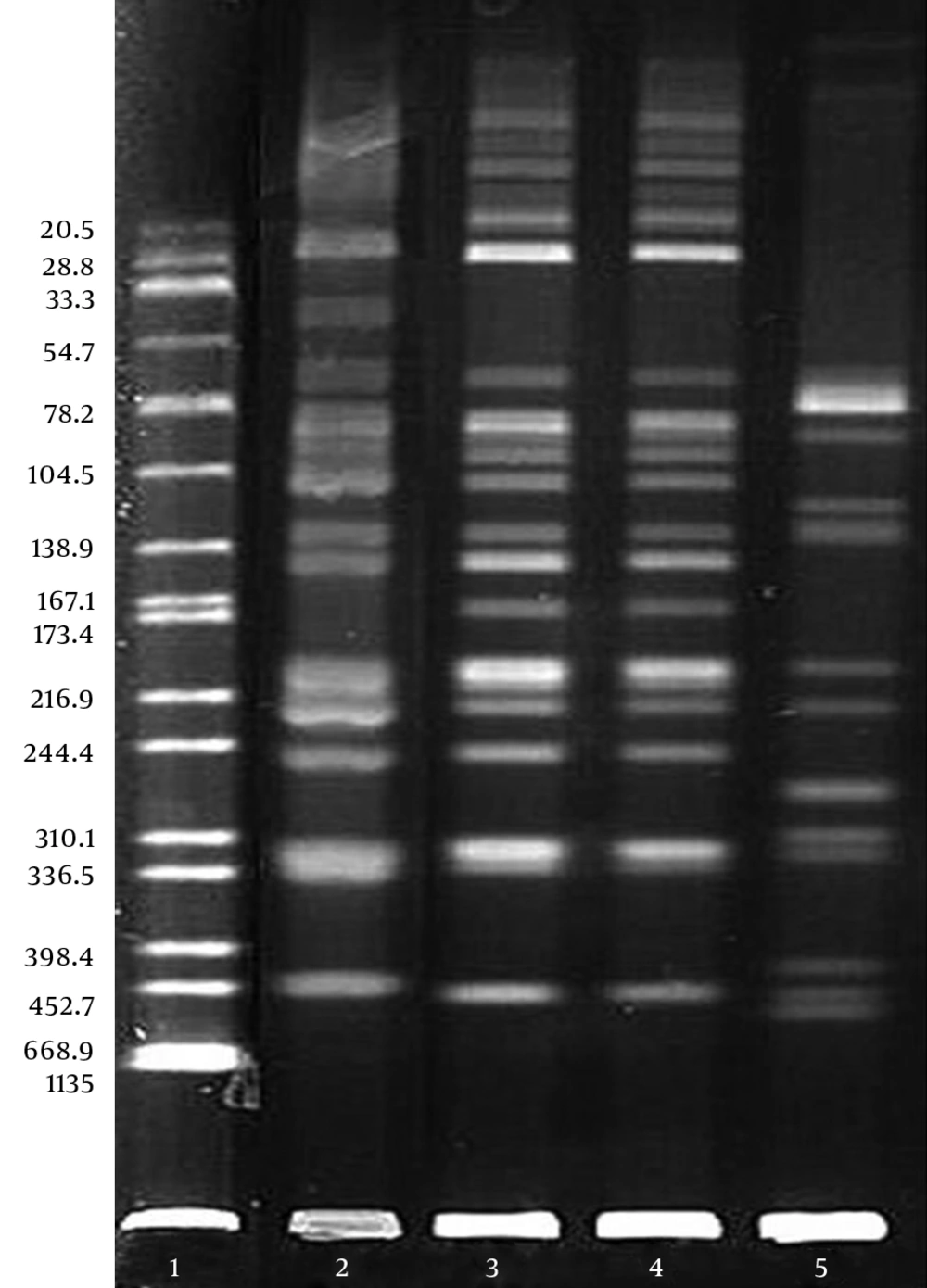
Four strains (blood samples in Cases 1, 2, and 3; catheter sample in Case 2) of clinically isolated R. mannitolilytica were used for the pulsed-field gel electrophoresis (PFGE) homology analysis of chromosomal DNA. The bacterial strains were digested overnight with protease K (Roche Applied Science, Mannheim, Germany) at 50°C, followed by digestion with the restriction endonuclease Spe I (Takara, Dalian, China) at 37°C for 14 hours. The primers were designed according to the literature (10-12), and the sequence of PCR primers were: 5’-3’ forward: GGGAAAGCTTGCTTTCCTGCC; 5’-3’ reverse: TCCGGGTATTAACCAGAGCCAT. The reaction system was: 95°C for 5 minutes, 94°C for 40 seconds, 55°C for 40 seconds, 72°C for 1 minute (30 cycles), and 72 °C for 5 minutes.
The DNA samples were subjected to electrophoresis using the CHEF Mapper XA PFGE system (Bio-Rad, CA, USA) at 14°C and 6 v/cm, with a pulse angle of 120° and pulse time of 5 - 35 seconds for 19 hours. After staining with ethidium bromide, the results were observed using a gel image analysis system and analyzed according to the standard determination of PFGE bands developed by Tenover et al. (13). Salmonella H9812 was used as the molecular-weight control.
3.6. Environmental and Item Samples
After the third patient developed the disease, according to the epidemiological characteristics of nosocomial infections, samples were taken from ventilator tubes, ventilator water, tap water, water used for drug injections, disinfectant solutions, medical-record folders, and the fingers of medical care personnel in the Department of Minimally Invasive Surgery. A total of 34 samples were collected, then inoculated in blood agar plates for culturing.
4. Results
4.1. Strain Identification and Drug Susceptibility Testing
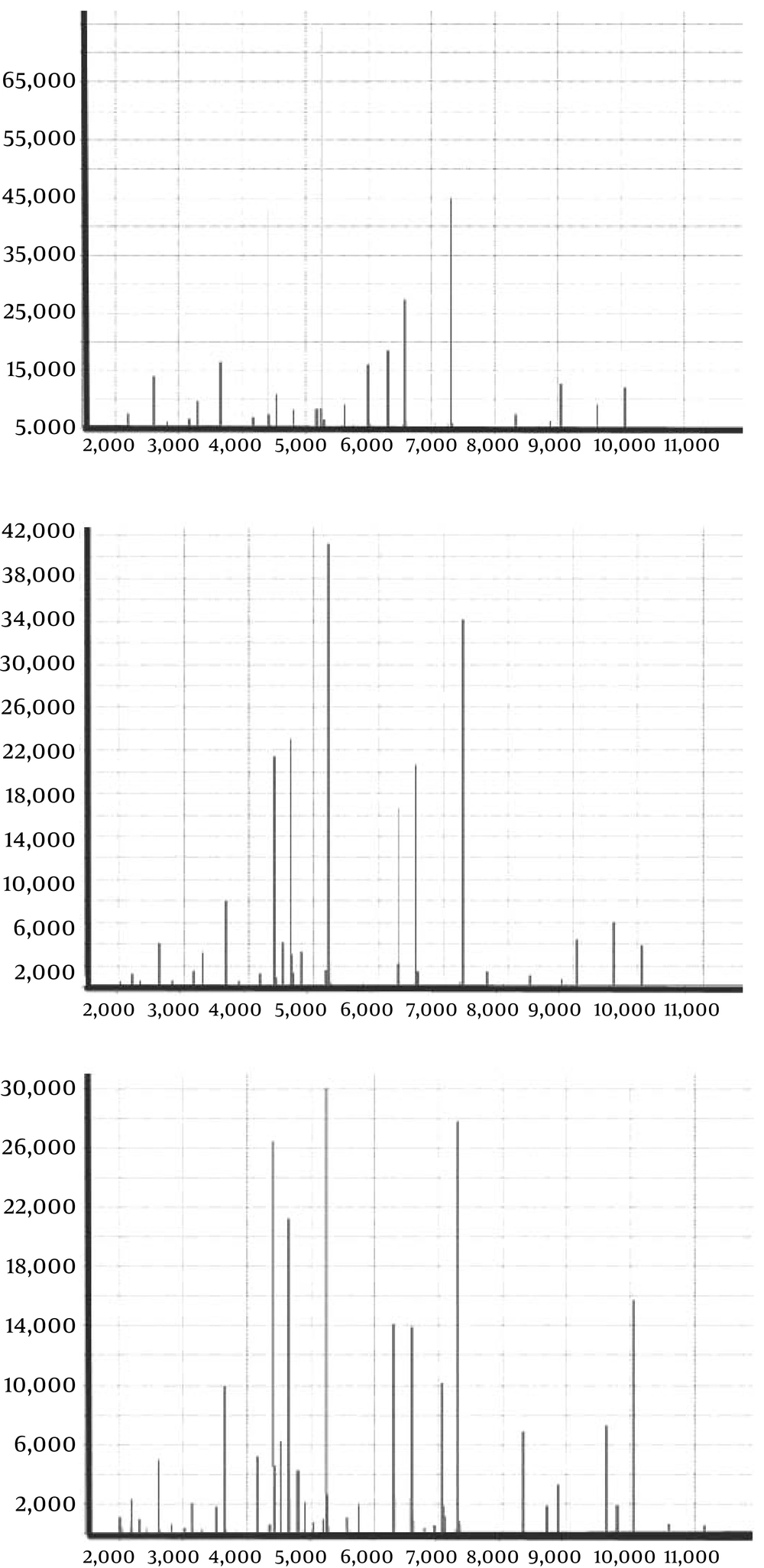
Bloodstream infections were confirmed in all 3 patients according to the operating instructions of the VITEK-2 bacterial identification system. The bacterial strain was identified as R. mannitolilytica using the VITEK Compact 2, and the biological code was 4203601303500141. The identification rate was > 95%. The identification results from the VITEK MS also indicated R. mannitolilytica; the coincidence rate was 99.9% (Figure 1).
The drug susceptibility test results of 4 strains of R. mannitolilytica are shown in Table 2. The strain isolated from Case 1 was sensitive to the majority of the tested antimicrobial agents, which is consistent with the characteristics of the wild-type strain. This patient lived in the mountains and had hematemesis before visiting our hospital for medical treatment. The drug-resistant spectra of Cases 2 and 3 were similar; these strains were resistant to multiple drugs and sensitive only to cotrimoxazole, ceftriaxone, and Tazocin, which is consistent with the characteristics of the drug-resistant strain in nosocomial infections.
| Antimicrobial drug | Case 1 | Case 2 | Case 3 | |
|---|---|---|---|---|
| Blood | Blood | Catheter | Blood | |
| Cotrimoxazole | S (≤ 20) | S (≤ 20) | S (≤ 20) | S (≤ 20) |
| Ampicillin/sulbactam | S (≤ 2) | I (16) | I (16) | R (≥ 64) |
| Piperacillin/tazobactam | S (≤ 4) | S (≤ 4) | S (≤ 4) | S (≤ 4) |
| Ceftriaxone | S (4) | S (2) | S (2) | S (4) |
| Amikacin | S (4) | R (≥ 64) | R (≥ 64) | R (≥ 128) |
| Gentamycin | S (2) | R (≥ 16) | R (≥ 16) | R (≥ 256) |
| Tobramycin | S (2) | R (≥ 16) | R (≥ 16) | R (≥ 64) |
| Ampicillin | R (≥ 32) | R (≥ 32) | R (≥ 32) | R (≥ 64) |
| Cefazolin | R (32) | R (≥ 64) | R (≥ 64) | R (≥ 64) |
| Ceftazidime | S (2) | R (≥ 64) | R (≥ 64) | R (≥ 64) |
| Cefepime | R (≥ 1) | R (32) | R (32) | R (8) |
| Aztreonam | S (8) | R (≥ 64) | R (≥ 64) | R (≥ 256) |
| Imipenem | R (≥ 1) | R (≥ 16) | R (≥ 16) | R (≥ 16) |
| Ciprofloxacin | S (0.5) | R (≥4) | R (≥4) | R (≥ 32) |
| Levofloxacin | S (0.5) | R (≥ 8) | R (≥ 8) | R (≥ 64) |
| Nitrofurantoin | R (256) | R (≥ 512) | R (≥ 512) | R (≥ 512) |
Results of in Vitro Drug-Susceptibility Testing of R. mannitolilytica
4.1. Growth Testing
Through growth testing, we found that the nutritional requirements of R. mannitolilytica were very low, as it could survive for a long time in nutrient-deficient distilled water. However, the 4 strains of R. mannitolilytica only survived for 5 weeks at 4°C, while they could survive for more than 26 weeks at 37°C or room temperature.
4.2. Homology Analysis
The DNA fingerprint analysis of the 4 strains of R. mannitolilytica was performed using the PFGE typing technology. A comparison of the DNA fingerprint profiles of the 4 bacterial strains demonstrated that these 3 patients were infected with different clonal strains of the same bacteria. The PFGE typing result is shown in Figure 2. PFGE results revealed that R. mannitolilytica strains from the blood and catheters of Case 2 came from the same clone (Lanes 3 and 4); all separated strains can be confirmed at the band around 398 Kb.
4.3. Environmental Cultures and Control Measures for Infection
Ralstonia mannitolilytica was not cultured from all of the collected environmental samples. However, no similar case has occurred again after the implementation of active control measures for nosocomial infections, including changing the batch water used for injection and disinfection solutions, complete disinfection of ventilator tubes, shortening the changing time of the humidifier, complete disinfection of possibly contaminated areas using chlorine-containing disinfectants and ultraviolet rays, strengthened education, and increased hand-hygiene compliance of medical care personnel.
5. Discussion
Ralstonia spp. is a non-pathogenic environmental microbe; clinical infection with this species is very rare (6, 14). However, the development of modern medical care, the non-standard use of broad-spectrum antibiotics, and the extensive use of various immunosuppressants has caused increased rates of such infections, mostly R. pickettii. There are fewer clinical infections caused by R. mannitolilytica; however, it often results in more severe conditions, such as septicemia, recurrent meningitis, myelitis, and peritonitis (6). An R. mannitolilytica outbreak occurred in 22 states in the USA in 2005, in which 8 cases were lung infections and 30 had colonizations. The investigation results suggested that this outbreak was caused by contaminated oxygen-delivery devices (3). In 2013, Israel also reported R. mannitolilytica infections in infants due to the use of the Vapotherm 2000i oxygen delivery device (5). Grobner et al. (15) reported 5 cases of catheter-related R. mannitolilytica-induced bacteremia in leukemia patients in 2007. The contamination source was not finally identified, but it was suggested that intravenous solutions were contaminated and that the use of immunosuppressants and permanent indwelling intravenous devices were the risk factors for this bacterium.
In the present study, the 3 patients were older in age, their immune systems were weaker, and the duration of invasive mechanical ventilation and deep vein catheterization was longer; therefore, these patients had the risk factors for infection with this bacterium. Their initial clinical symptoms were chills and fever, followed by shock symptoms, such as shortness of breath, increased heart rate, rapidly decreased blood pressure, and indifference; these symptoms were basically consistent with descriptions in the literature (4, 15). Laboratory tests indicated a progressive reduction in the numbers of WBCs and platelets, an increase in the non-specific indicator CRP, and significant increases in specific indicators of bacterial infection, such as endotoxins and PCT; Cases 1 and 3 had increased levels of brain natriuretic peptide (BNP) in the presence of heart failure symptoms.
The PFGE results revealed that both strains in Case 2 were from the same clone; therefore, it could be determined that Case 2 had a catheter-related bloodstream infection. One report has demonstrated that R. mannitolilytica can form a biofilm on plastic catheters (7). The 3 patients in this study all had postoperative catheterizations; however, the bacterium was not cultured from the catheters of Cases 1 and 2; this phenomenon might be associated with slow bacterial growth, a low amount of bacteria on the catheters, the methods used for placing and removing the catheters, and/or the untimely submission of specimens. On a retrospective review of the medical history of Case 1, we speculated that the bacteria might have colonized in the respiratory or digestive system, thereby causing an endogenous infection during the surgical process.
In addition, the growth testing indicated that these bacteria thrived in a warm and humid environment. These bacteria also have the ability to pass through a 0.2-m filter (16, 17) and are disinfectant-resistant (18). These characteristics are consistent with domestic and international reports. The use of ventilators, oxygen delivery devices, venous catheterization, and contaminated medical water are the high-risk factors for infection with R. mannitolilytica (1, 5, 15, 18). The PFGE detection assay showed that the DNA fingerprint profiles of the 3 bacterial strains were not the same, indicating that they were isolated nosocomial infection events. This bacterium was not isolated in the environmental specimens, and active nosocomial infection-control measures effectively prevented any reinfections with it. Former reports indicated that R. mannitolilytica is prevalent in many different types of water supplies (6, 19). Inconsistent with these reports, we were inclined to define ectopic infections those caused by opportunistic pathogens, but further research is needed to prove this theory.
Currently, there are no clear treatment guidelines or CLSI breakpoints for identifying R. mannitolilytica infections. In the course of treatment, we advocate using drug-susceptibility testing to adjust the use of antimicrobial agents. In addition, cotrimoxazole, ceftriaxone, and piperacillin/tazobactam are recommended for empirical treatment. Microbiological-detection personnel should pay attention to the slow growth of this bacterium. It is suggested that the growth condition should be determined after 48 hours of culturing; otherwise, a missed diagnosis is likely to occur. The problems caused by this bacterium occur rapidly and disease progression is fast; therefore, R. mannitolilytica infections should draw sufficient attention from clinical physicians and bacteriology workers in order to respond to the resulting severe consequences.