1. Introduction
Leishmaniasis has been known as an endemic disease since ancient times in Iran. So far, several researches have been carried out to identify proteins and antigens that stimulate the immune system, especially cellular immunity. Some of these proteins and immunogenic antigens have been suggested to prevent the disease through vaccine use (1).
Macrophages, as host of the parasite, have a significant role in immunity against Leishmaniasis. The mechanism for removal of Leishmania is done through the activation of macrophages, by virtue of toxic oxygen metabolites, including superoxide anion O2, hydrogen peroxide (H2O2) and nitric oxide (NO) (2) or by proteolytic enzymes such as neutrophil elastase releasing enzyme (3). For macrophages, activating a number of stimuli can induce morphological changes, biochemical and functional characteristics of macrophages. Activated macrophages produce many cytokines such as tumor necrotising factor (TNF)-α, IL-6, IL-18, IL-12 and IFN-γ. interleukin (IL)-12 acts as an effective adjuvant and a prerequisite for type 1 immune response in most intracellular infections (4). Among cytokines, IFN-γ has an important role, so that if the cytokine or its receptor is destroyed, the mice were not be able to limit the growth of Leishmania major in the body, which may lead to death of the mice (5, 6).
Other cytokines such as IL-2, IL-4 and IL-7 in combination with IFN- γ, with synergistic effect, cause macrophages activity that leads to Leishmania removal. The permanent production of IL-4 usually causes persistence of the disease (7). Interleukin-4 was shown to play an important role in mediating susceptibility to Leishmania infection by down-regulating the expression of protective Th1-associated cytokines IL-12 and IFN-γ and by inhibiting NO production and parasite killing by macrophages (8). Interleukin-4-deficient BALB/c mice have shown that IL-4-mediated exacerbation of CL is dependent upon the particular strain of Leishmania (9).
Research on leishmanial vaccine has been carried out by some researchers using Iranian strain of L. major (10-13). In the present work, we tried to introduce a new DNA Leishmanial vaccine candidate. This type of vaccine could be a good option for use as it is safe, does not contain any infectious agent, effectively stimulates different extents of the immune response, and can be produced in large volumes at a much lower cost than some traditional vaccines.
2. Objectives
In the present study, the cellular immunogenicity of a DNA vaccine candidate (KMP11-NTGP96-GFP fusion) in induction of Th1 platform immune response was evaluated in susceptible BALB/c mice.
3. Methods
3.1. Parasite and Plasmids
The Iranian strain of Leishmania major (MRHO/IR/75/ER) was kindly provided by Tehran University of Medical Sciences, Iran. Promastigotes were cultured in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS, Sigma-Aldrich, Germany) and incubated at 24 ± 1°C. A pBluescript-Gp96 plasmid containing the Xenopus Gp96 DNA (accession number AY187545, 2552 bp) was kindly provided by Dr. Bolhassani (Pasteur Institute of Iran, Tehran, Iran). Escherichia coli strains TOP10 were obtained from Novagene Co., and pJET1.2/blunt cloning vector as well as pEGFP-N1 expression vector were obtained from Fermentas and Invitrogen Co., respectively.
3.2. Genomic DNA Extraction
Genomic DNA of L. major promastigotes was extracted by commercial DNA Extraction kit (Bioneer, Korea), according to the manufacturer’s protocol. Quality and purity of extracting DNA were assessed by electrophoresis on 1% agarose gel and spectrophotometrically.
3.3. Primer Design and POLYMERASE Chain Reaction Amplification
3.3.1. KMP-11
A pair of oligonucleotide primers was designed based on the KMP- II gene sequences (279 base pair) that were recorded in articles as follows:
Forward primer: 5’- TCT AGA ACC ATG GCC ACC ACG TAC GAG GAG-3’ that ACC ATG: Kozak sequence and TCT AGA: XbaI cut site.
Reverse primer: 5’-GGA TCC TTA CTT GGA TGG GTA CTG CGC AGC-3’ that GGA TCC: BamHI cut site and without stop codon.
The PCR reaction mixture was as follows: the 50-µL PCR mixture comprised of 5 µL of parasite genomic DNA as the template (100 ng), 200 µM of each dNTPs (deoxynucleoside triphosphates, Fermentas), 5 units of Pfu DNA polymerase (Vivantis) with its 1X ViBuffer A, 1.5 mM MgCl2, and 20 picomoles of each of the forward and reverse primers.
The polymerase chain reaction procedure was as follows: 95°C for two minutes as initial denaturation, 35 cycles at 94°C for 30 seconds, 58°C for 30 seconds, 72ºC for 30 seconds and then 72°C for 10 minutes as final extension.
3.3.2. NTgp96
The forward and reverse primers for amplifying the NTgp96 of Xenopus Gp96 DNA (accession number AY187545, 1014 base pair) were as follows:
Forward primer: 5'- CGG GGA TCC GAA GAT GAC GTT GAA -3' that GGA TCC: BamHI cut site.
Reverse primer: 5'- AT GGT ACC TTT GTA GAA GGC TTT GTA-3' that GGT ACC: KpnI cut site.
The PCR reaction mixture was as follows: a 50-µL PCR mixture that comprised of 1 µL of pBluescript-Gp96 plasmid (100 ng), 200 µM of each dNTPs (deoxynucleoside triphosphates, Fermentas), 5 units of Pfu DNA polymerase (Vivantis) with its 1X ViBuffer A, 1.5m M MgCl2, and 20 picomoles each of the forward and reverse primers. The following amplification program was used: 95°C for five minutes as initial denaturation, 30 cycles at 95°C for one minute, 62°C for two minutes and 72°C for 1.5 minutes and then 72°C for 20 minutes as final extension. Finally, the PCR products were analyzed by electrophoresis on 1.2% (w/v) agarose gel. KMP-11 and NTgp96 gene fragments were purified from gel using a gel purification kit (Bioneer, Korea).
3.4. Cloning of KMP-11 and NTgp96 into pJET1.2/blunt Cloning Vector
The KMP-11and NTgp96 PCR products bands were cut under long (365) UV wavelength and recovered by gel purification kit (Vivantis). Recovered DNAs were ligated into pJET1.2 cloning vector at 22°C for an hour and then at 4°C overnight with T4 DNA ligase (Fermentas Co.). The ligation products were transformed into E. coli TOP10 strain competent cells and dispersed onto lysogeny broth (LB) agar plates containing 100 µg/mL ampicillin. After overnight incubation at 37°C, colonies on the agar plate that contained recombinant plasmids were detected. For confirmation, PCR amplifications were performed in these colonies using primers specific for KMP-11and NTgp96 genes and pJET vector and colonies containing the recombinant plasmid were selected. Recombinant plasmids were extracted by Vivantis plasmid extraction kit and digested by XbaI / BamHI (for KMP-II), and BamHI/ KpnI (for NTgp96) restriction enzymes (Fermentas Co.®) for digestion confirmation.
3.5. Subcloning of KMP-11 and NTgp96 into pEGFP-N1 Expression Vector
The recombinant plasmids pJET- KMP-11 and pEGFP-N1 were digested with XbaI and BamHI restriction enzymes. Also the recombinant plasmids pJET-NTgp96 and pEGFP-N1 were digested by BamHI and KpnI restriction enzymes. The products of digestion were analyzed by electrophoresis on 1.2% agarose gel, and the digested bands of KMP-11 fragment (279 bp), NTgp96 fragment (1014 bp) and pEGFP-N1 were purified by gel purification kit (Vivantis Co.). The KMP-11 and NTgp96 genes were ligated into digested pEGFP-N1 expression vector using T4 DNA ligase enzyme. Recombinant plasmids pc-KMP-11 and pc-NTgp96 were transformed into E. coli TOP10 strain competent cells and dispersed onto LB agar plates containing 100 µg/mL of ampicillin and incubated at 37°C overnight. Afterward, PCR amplifications were performed for colonies on the agar plate using specific primers of KMP-11 and NTgp96 genes, and colonies containing the recombinant plasmid were selected. Then, recombinant plasmids were extracted by Vivantis plasmid extraction kit and digested by XbaI / BamHI (for KMP-11) and BamHI/ KpnI (for NTgp96) restriction enzymes (Fermentas Co.).
3.6. Design of the pEGFP-N1- KMP 11-NTGP96 Fusion Construct
The recombinant plasmids pEGFP-N1- KMP 11 and pJET-NTgp96 were digested by BamHI and KpnI restriction enzymes. The products of digestion were analyzed by electrophoresis on 1.2% agarose gel, and the digested bands of NTgp96 fragment (1014 bp) and pEGFP-N1- KMP 11 were purified by gel purification kit (Vivantis Co.). NTgp96 genes were ligated to digested pEGFP-N1- KMP 11 using T4 DNA ligase enzyme. Recombinant plasmids pEGFP-N1- KMP11-NTGP96 were transformed to E. coli TOP10 strain competent cells and dispersed onto LB agar plates containing 100 µg/mL of ampicillin and incubated at 37°C overnight. Next, colonies on the agar plate that contained recombinant plasmids were detected. For confirmation, PCR amplifications were performed for these colonies using primers specific for KMP-11and NTgp96 genes and colonies containing the recombinant plasmid were selected. Recombinant plasmids were extracted by Vivantis plasmid extraction kit and digested by XbaI / BamHI (for KMP-11), BamHI/ KpnI (for NTgp96) and XbaI / KpnI (for KMP 11 -NTGP96) restriction enzymes (Fermentas Co.) for digestion confirmation.
3.7. Mice, Immunization Schedules
Female inbred BALB/c mice, six week-old were purchased from Razi Vaccine and serum research institute, Karaj, Iran. They were housed in clean cages and fed ad libitum. The immunization experiments were carried out in four groups of mice (n = 15 at each group) and all tests were done in triplicates. The first group received phosphate buffered saline (PBS) only; Group 2 was immunized with pEGFP-N1; group 3 vaccinated with pEGFP-KMP11; and group 4 vaccinated with pEGFP-KMP11-GP96 (FUSION). All groups were immunized with 100 µg of the materials three times (0, 21 and 42 days) intramuscularly.
3.8. Cytokine Assays
To determine the levels of IFN-γ and IL-4, in each experimental group, five mice were sacrificed before and also seven weeks after last immunization and their spleens were removed and homogenized in PBS. After erythrocytes lysis using ACK lysis buffer (0.15 M NH4Cl, 10 mM KHCO3 and 0.1 mM Na2-EDTA), splenocytes were washed with PBS and resuspended in RPMI-10% FCS. Cells were then seeded at a density of 3.5 × 106 cells/mL in the presence of L. major Freeze/Thawed antigen (25 mg/mL). Medium alone was used as the negative control. Plates were incubated for 72 hours at 37°C in 5% CO2 humidified atmosphere for IFN-γ and IL-4 measurement. The IFN- γ and IL-4 production in supernatants of splenocyte cultures was measured by the enzyme linked immunosorbent assay (ELISA) kits (U-CyTech, Netherlands), according to the manufacturer’s instructions. All experiments were run in triplicates.
3.9. Ethics Statement
This research was carried out in accordance with the recommendations in the Guide for the care and use of laboratory animals of the Tarbiat Modares university. All animal experiments, including maintenance, handling and blood collection was approved by the institutional animal care and research advisory committee of Tarbiat Modares University, based on the specific national ethical guidelines for biomedical research issued by the research and technology deputy of ministry of health and medicinal education of Iran.
3.10. Statistical Analysis
Statistical analysis was performed using SPSS version 18 and one way analysis of variance (ANOVA) (Multiple-comparison Tukey post Hoc test) and Student’s t-test was employed to assess the significance of the differences between the mean values of control and experimental groups. Differences were considered statistically significant when P < 0.05. Data represent the mean values ± standard error of the mean (SEM) of three independent experiments
4. Results
The results of cellular immune response by measuring cytokines are presented in Tables 1 and 2. Table 1 shows the cytokine IFN-γ concentration, before and seven weeks after the immunization in different groups. According to the data in Table 1 by comparing the results of the level of cytokines IFN-γ before and seven weeks after immunization among different groups, the results showed that after immunization the level of IFN-γ in the groups that received pEGFP-KMP and pEGFP-KMP-GP96 (FUSION) were increased significantly and had a significant difference with PBS and pEGFP-N1 groups (P < 0.05).
| Group Immunized With | Before Immunization IFN-γ, Pg/mL | After Immunization IFN-γ, Pg/mL | P Value ≤ 0.05 |
|---|---|---|---|
| PBS | 1.56 ± 0.043 | 2.48 ± 0.036 | 3, 4 |
| pEGFP-N1 | 1.68 ± 0.037 | 3.27 ± 0.41 | 3, 4 |
| pEGFP-KMP | 1.73 ± 0.21 | 11.12 ± 0.76 | 1, 2, 4 |
| pEGFP-KMP-GP96 (FUSION) | 1.64 ± 0.18 | 13.30 ± 0.75 | 1, 2, 3 |
| Group Immunized with | Before Immunization IL4, Pg/mL | After Immunization IL4, Pg/mL | P Value ≤ 0.05 |
|---|---|---|---|
| PBS | 9.40 ± 0.47 | 10.14 ± 0.62 | 3, 4 |
| pEGFP-N1 | 10.27 ± 0.28 | 10.11 ± 0.23 | 3, 4 |
| pEGFP-KMP | 9.40 ± 0.15 | 2.85 ± 0.16 | 1, 2 |
| pEGFP-KMP-GP96 (FUSION) | 10.53 ± 0.23 | 2.77 ± 0.18 | 1, 2 |
According to the data in Table 2 by comparing the results of the level of cytokine IL4 before and seven weeks after immunization among different groups, the result showed that after immunization the level of IL4 in the groups that received pEGFP-KMP and pEGFP-KMP-GP96 (FUSION) decreased significantly and had a significant difference with PBS and pEGFP-N1 groups (P < 0.05).
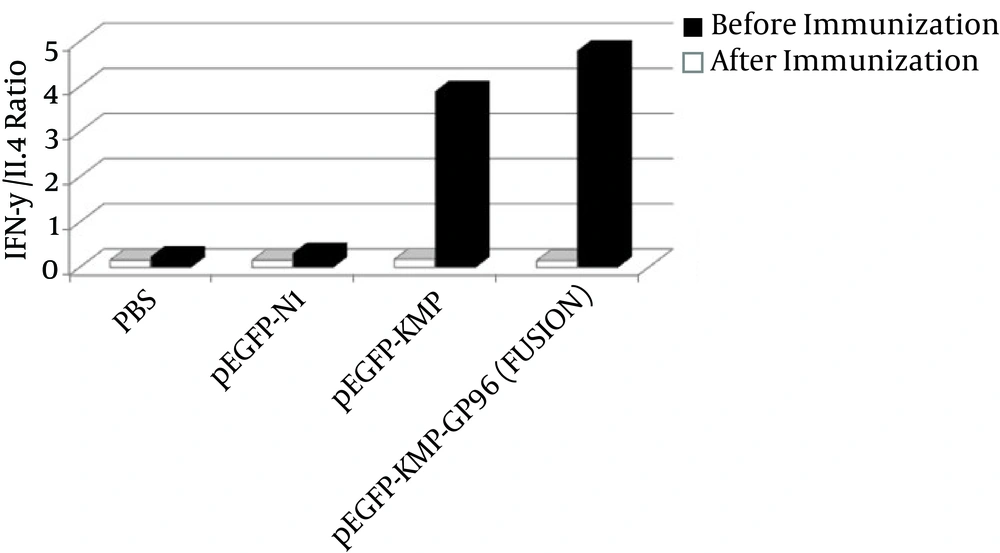
According to Figure 1, after immunization, the proportion was higher in the group receiving the fusion gene, which indicates that the cellular immunity in this group was higher than others.
5. Discussion
Cell-mediated immunity plays a decisive role in Leishmaniasis. A promising vaccine against Leishmania could stimulate the Th1 immune response. Depending on the immune status of the host, two phenomena may occur once Leishmania parasites enter the body: in the first case, in animals that are genetically resistant to the parasites, Th1 cell induction occurs. These cells produce IL2 and IFN γ, followed by a parasitic elimination. In the second case, which occurs in susceptible animals, the Th2 cells are induced. They produce IL4 and IL-10 that stimulate the parasites proliferation and disease progression (14). Usually, antigens with known epitope accompanied with cellular immunity are a good choice for immunogenicity. According to Das et al. (2014), many indicator epitopes are associated with the KMP-11 genes that can pitch our goal to provide T-cells stimulation (15).
In the recent years, antigens like LACK, LeIF, TSA, LmSTI1, H1, CPA + CPB, KMP-11 and NH36 have been evaluated for Leishmania vaccine. These antigens have been tested in animal models and have been promising for third generation vaccines against Leishmania. In the study of Gurunathan et al. (1997), immunization with DNA encoding the immunodominant LACK parasite antigen by stimulating the production of IFN-γ caused Th1 immune response, which finally conferred a protective immunity to mice infected with L. major (16). In a study conducted by Ramos et al. (2009) that used DNA-LACK / MVA-LACK, results showed prime-boost strategy, reducing clinical symptoms and parasite load in the liver, increasing the activity of Th1 cells, stimulation of Th1 response and decreased Th2 response against canine visceral Leishmaniasis (17).
In the study of Campos-Neto et al. (2002), immunization with plasmid DNA encoding TSA/LmSTI1 Leishmanial fusion proteins confers protection against L. major infection in susceptible BALB/c mice (18). In the study of Rafati et al. (2005), immunization with a combination of DNA and cysteine protease type I and II protein of the parasite, increased IgG2 synthesis, lymphocyte proliferation, IFNγ / IL-10 secretion and DTH response (19). In a study by Basu et al. (2005) DNA immunization of hamsters with KMP-11, caused a combination of cytokine production of Th1 (IFN-γ, TNF-(and IL-12 cytokines) and Th2 (decreased production of IL-10 and IL-4) and an increase in production of nitric oxide (NO) and protection against visceral Leishmaniasis (20). While in the study of Rodríguez-Cortés et al. (2007), immunization with plasmid DNA encoding KMPII, TRYP, LACK and GP63 did not protect dogs against Leishmania infantum experimental challenge (1). In a study conducted by Aguilar et al. (2005), L. donovani DNA vaccines of NH36, showed better prophylactic efficacy than recombinant protein of NH36 plus FML and Saponin against cutaneous and visceral Leishmaniasis in mice. Therefore, the DNA vaccine has been introduced as a good candidate for a cross-immunity against Leishmania species (21).
Comparing the level of cytokine IFN-γ among different groups, our results revealed that this cytokine in the group, which received pEGFP-KMP-GP96 (FUSION), showed of a significant increase after immunization. This result represents an appropriate response to the immunization and the persistence of immune memory. In other words, the synergistic combination of KMP-11 and GP96 gene fusion is able to stimulate sufficient IFN-γ production. Therefore, this option can be a good candidate for a vaccine against Leishmaniasis.
Interleukin-4 is an indicator of Th2 cell response and is produced by basophils, mast cell, Th2 and NK. They inhibit IL-12 production and have inhibitory effects on the activity of macrophages (22). According to our results, the level of cytokine IL-4 was decreased after vaccination in groups immunized with pEGFP-KMP11-GP96 (fusion) and pEGFP-KMP11. In addition, the IFN-γ and IL-4 ratio in pEGFP-KMP11-GP96 (fusion) had higher values than the other groups after immunization. In general, the group vaccinated with fusion, is our best option to stimulate active factors in establishing protective immunity against cutaneous Leishmaniasis.
To better confirm the results of this study, an immune protection assessment of the constructed fusion against cutaneous Leishmaniasis in BALB/c mice will be a good subject for future research.