1. Background
Pseudomonas aeruginosa is the most common bacterial pathogen in serious nosocomial infections such as pneumonia, urinary tract infection, surgical site infections and sepsis (1). Pseudomonas aeruginosa could potentially become resistant to all classes of the antibiotics used to treat gram-negative associated nosocomial infections including β-lactams, aminoglycosides and fluoroquinolones (2).
The development of resistance to β-lactam in this opportunistic pathogen is driven by several mechanisms such as production of β-lactamase, overproduction of efflux systems and reduced permeability (1). The OXA-type β-lactamases are widespread and have been mostly identified in clinical isolates of P. aeruginosa, which commonly confer resistance to cephalosporins or carbapenems (3). The enzymes encountered most frequently are carbenicillinases of the PSE (CARB) group (Ambler class A) and oxacillinases (Ambler class D). Classical PSE and OXA enzymes confer resistance to carboxypenicillins and ureidopenicillins (4). Extended spectrum beta-lactamase (ESBL)-producing P. aeruginosa have been reported from around the world and have recently been shown to have a specific geographical distribution; for example, OXA-10 is widespread in Turkey or in the Kerman city of Iran (2010). blaOXA-10 was detected in 92.7% (4-8).
Rep-PCR analyzes apply primers targeting highly conserved repetitive sequence elements in the bacterial genome. One of such groups of repetitive elements is the Enterobacterial repetitive intergenic consensus (ERIC) sequences communal to gram-negative enteric bacteria (9). The products of the ERIC-PCR, with chromosomal DNA of dissimilar bacterial strains as template, were established to generate very characteristic patterns when separated on agarose gels (7). Thus, relatedness of the isolates was also explored using ERIC-PCR.
2. Objectives
The aims of the present study were determination of the antibiotic susceptibility pattern and genotyping of P. aeruginosa isolated from four Iranian hospitals in Tabriz, to investigate the prevalence of OXA producer isolates and mechanism of resistance in P. aeruginosa isolates.
3. Methods
3.1. Bacterial Isolates
During a 12-month period, from October 2013 to September 2014, 151 non-duplicated clinical isolates were collected consecutively from three hospitals including Emam Reza hospital, Sina hospital and Shahid Madani hospital, in the city of Tabriz, Iran. The isolates were obtained from blood, sputum, urine, respiratory tract, wound and cerebrospinal fluid (CSF) from hospitalized patients. Gram staining and standard biochemical tests were performed for identification of isolates at the microbiology department of Tabriz University of Medical Sciences (10).
3.2. Antimicrobial Susceptibility Testing
The disk diffusion susceptibility testing was performed according to the clinical and laboratory standards institute (CLSI) guidelines. Antibiotic discs (MAST diagnosis, England) used in this study included: imipenem (10 μg), colistin (10 μg), amikacin (30 μg) cefepime (30 μg) ceftazidime (30 μg), tobramycin (30 μg), gentamicin (30 μg), ciprofloxacin (5 μg), polymyxin B (300 units), gatifloxacin (5 μg) and piperacillin (100 μg) (11). All tests were done in Muller Hinton Agar (Merck, Germany). Escherichia coli 25922 and P. aeruginosa ATCC 27853 were used as standard strains for quality control in Antibiogram.
Multi drug resistant (MDR) identification: In this study MDR was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories (12).
3.3. Frequency of OXA Genes
DNA was extracted by sodium dodecyl sulfate (SDS) (Merck, Germany), proteinase K and cetyl trimethylammonium bromide (CTAB) (Merck, Germany) method (13). The presence of Ambler class D β-lactamases genes was detected by PCR. Three primer pairs (Bioneer, Korea) screening OXA (blaOXA-group I, blaOXA-group II and blaOXA-group III) and two primer pairs for OXA-1 and OXA4 genes were used in the PCR (Table 1). All PCR amplifications were done by Sinagen Mastermix (Sinagene, Tehran, Iran). The amplified products were analyzed by electrophoresis on 1% agarose gel containing ethidium bromide (1 mg/mL) at 70 V/cm for 90 minutes (14), and were detected by UV trans-illumination. The size of the fragment was determined by comparing with 100 bp DNA ladder plus size marker (13).
| Primer and Gens Name | Sequence (5′to 3′) | Product Size, bp | Reference |
|---|---|---|---|
| OXA group I | 5′-TCA ACA AAT CGC CAG AGA AG-3′ | 276 | (4) |
| 5′-TCC CAC ACC AGA AAA ACC AG-3′ | |||
| OXA group II | 5′AAG AAA CGC TAC TCG CCT GC-3′ | 478 | (4) |
| 5′-CCA CTC AAC CCA TCC TAC CC-3′ | |||
| OXA group III | 5′-TTT TCT GTT GTT TGG GTT TT-3′ | 427 | (4) |
| 5′-TTT CTT GGC TTT TAT GCT TG-3′ | |||
| OXA-1 | 5′-AGC CGT TAA AAT TAA GCC C-3′ | 911 | (15) |
| 5′-CTT GAT TGA AGG GTT GGG CG-3′ | |||
| OXA-4 | 5′-TCA ACA GAT ATC TCT ACT GTT-3′ | 216 | (16) |
| 5′-TTT ATC CCA TTT GAA TAT GGT-3′ | |||
| ERIC | 5′-CACTTAGGGGTCCTCGA ATGTA-3′ | (13) | |
| 5′-AAGTAAGTGACTGGGGTGAGCG-3′ |
3.4. Enterobacterial Repetitive Intergenic Consensus-Polymerase Chain Reaction Typing
The genetic similarity of the isolates was detected by amplification of the Enterobacterial repetitive intergenic consensus sequence (ERIC-PCR). Primers (Biooner, Korea) shown in Table 1 were used for the amplification. The PCR amplification was carried out with an initial denaturation step at 94°C for seven minutes followed by 35 cycles of denaturation (94°C for 50 seconds), primers annealing (50°C for one minute) and extension at 72°C for 130 seconds with a final extension at 72°C for 12 minutes. Eight microliters of the amplification product was analyzed on 2% agarose gel stained with ethidium bromide. Pseudomonas aeruginosa ATCC 27853 was used as the standard strain for comparing with clinical samples. All data analysis was performed using a computer online software gelcompar_6. 6.11 for Windows (17). Chi-square test (or Fisher exact test) was performed for data analysis. P values below 0.05 were considered significant.
4. Results
We accessed 151 clinical isolates, which were obtained from different infection sites including urine (n = 56), wound (n = 53), respiratory tract (n = 21), blood (n = 12), sputum (n = 6) and CSF (n = 3). In the present study, high level resistance was observed to gentamicin (68%) whereas colistin and polymyxin B had lowest level resistance to these drugs was observed in 2.5% of isolates. Table 2 shows antibiotic resistance pattern of bacterial isolates in this study. In present study, multi-drug resistant (MDR) isolates were observed in 61.8% of all isolates.
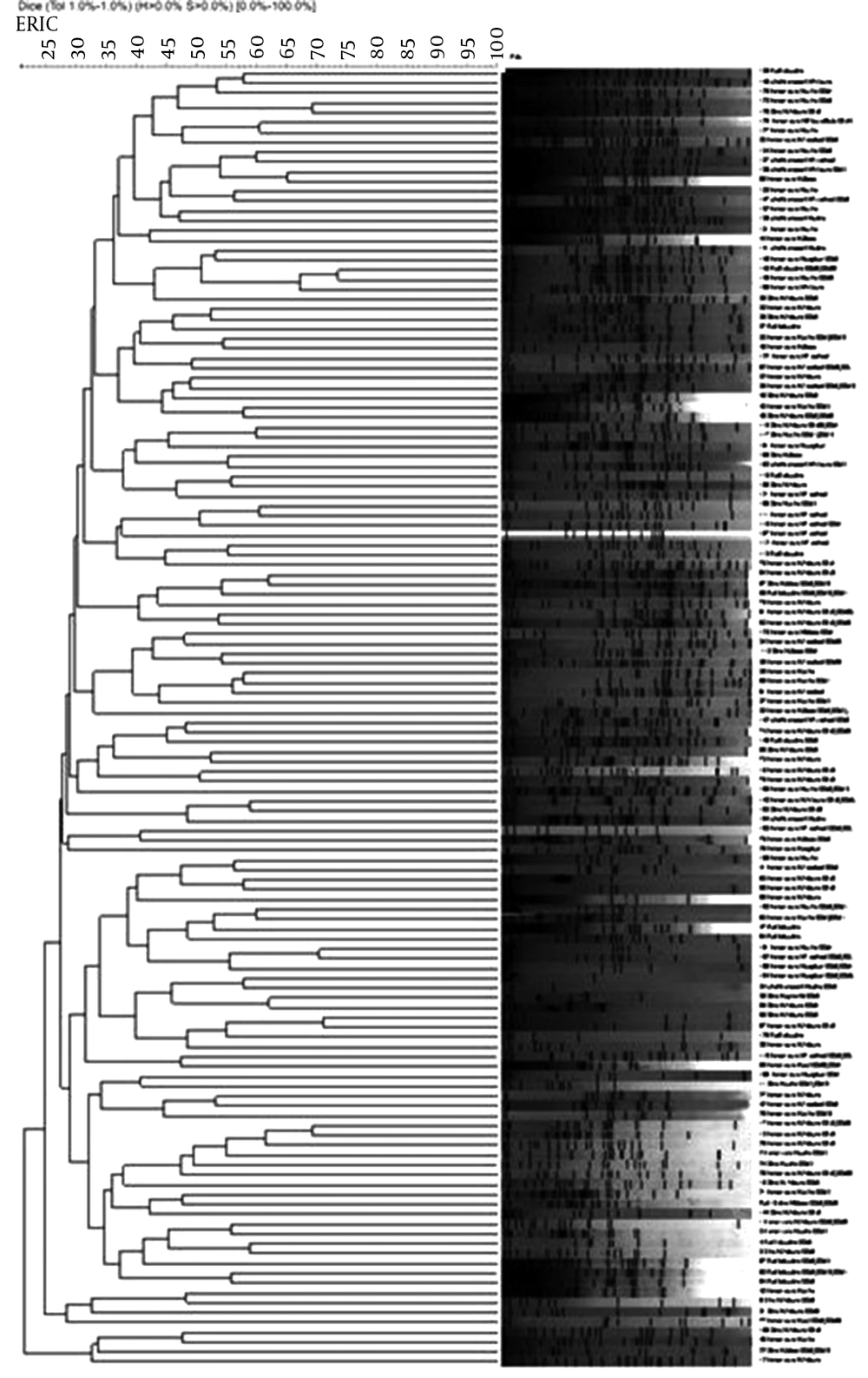
The PCR results showed that 56% of isolates carried the OXA group I genes. Twenty-six and 19% of isolates were PCR-positive for OXA group II and OXA Group III, respectively. The highest resistance rate to most antibiotics was observed in isolates of the OXA group I than group II or III genes. The results of the current study showed that all antibiotics, except polymyxinB and colistin, had significant correlation with OXA group I (P < 0.05) (Table 2). Moreover, ceftazidime was the least effective among isolates of OXA groups II and III and OXA-1 genes. Also in isolates that carried the OXA-4 gene, the rates of resistance to all tested antibiotics were less than 3%. The ERIC-PCR was used to distingue P. aeruginosa clinical isolates. Results indicated a banding pattern with sizes that ranged from approximately 50 pb to 2800 pb. Considering a Dice correlation coefficient of ≥ 0.95, 151 distinct ERIC-PCR patterns were acquired in 151 P. aeruginosa clinical isolates. All isolates (100%) were orphans with no relationship with other isolate (Figure 1).
| Antibiotics | GEN | CAZ | PIP | CPM | CIP | TOB | AK | IMI | GAT | PB | CL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Resistance of 151 | 103 (68) | 102 (67) | 101 (66) | 97 (64) | 95 (62) | 93 (61) | 91 (60) | 80 (52) | 43 (28) | 4 (2) | 4 (2) |
| OXAI | 68 (66) | 67 (65) | 67 (66) | 68 (70) | 66 (69) | 64 (68) | 65 (71) | 55 (68) | 24 (55) | 2 (50) | 4 (100) |
| OXAII | 21 (20) | 22 (21) | 19 (18) | 18 (18) | 20 (21) | 20 (21) | 20 (21) | 17 (21) | 4 (9) | 1 (25) | 3 (75) |
| OXAIII | 11 (10) | 12 (11) | 10 (9.9) | 10 (10) | 11 (11) | 10 (10) | 10 (10) | 8 (10) | 4 (9) | 0 | 0 |
| OXA1 | 13 (12) | 15 (14) | 13 (12) | 12(12) | 12 (12) | 12 (12) | 11 (12) | 11 (13) | 6 (13) | 1 (25) | 0 |
| OXA4 | 2 (1) | 1 (0.98) | 2 (15) | 1 (1) | 2 (2) | 2 (2) | 2 (2) | 2 (2) | 2 (4) | 0 | 0 |
Abbreviations: Gentamicin, GEN; ceftazidime, CAZ; piperacillin, PIP; cefepime, CPM; ciprofloxacin, CIP; tobramycin, TOB; amikacin, AK; imipenem, IMI; gatifloxacin, GAT; polymyxin B, PB; colistin, CL.
aValues are expressed as No. (%).
Nevertheless, according to the Figure 1 isolates number 128, 129; 148, 149; 101, 102; 95, 97 and 17, 18 could have been included in the same clusters and approximately had identical antimicrobial resistance profiles, but isolate source and OXA genes harboring was different. Therefore each isolate in the current study belonged to an orphan pattern.
5. Discussion
Pseudomonas aeruginosa is an important causative agent of human infection, especially in a host with compromised defense mechanisms (18). Pseudomonas aeruginosa is a leading cause of nosocomial infections via colonization of catheters, skin wounds, ventilator-associated pneumonia and it is also a cause of respiratory infections in individuals with cystic fibrosis (19). Pseudomonas aeruginosa has a capacity to expand resistance to several classes of antimicrobial agents; provoking the appearance of multi drug resistant (MDR) isolates (18). Unfortunately, choice of the most suitable antibiotics is complex, due to the capability of P. aeruginosa to extend resistance to multiple classes of antibacterial agents, even during the course of treating an infection (20). Similar to other studies carried out by Bayani et al., MDR was observed in 61.8% of all isolates. Lately, carbapenems are being administrated as the last resort antimicrobial to treat serious infections due to MDR P. aeruginosa. In the present study, 52% of isolates were imipenem resistant (21).
The most potent combination is clearly that of a carbapenemase-producing isolate commonly enriched by resistance to quinolones and aminoglycosides, leaving very limited options for antimicrobial treatment (16). Carbapenem-resistant P. aeruginosa is mainly an ICU pathogen, and one of the frequent causes of VAP. These infections are often related to poor outcomes. Treatment options consist of colistin, aminoglycosides, fluoroquinolones and fosfomycin while P. aeruginosa is intrinsically resistant to tigecycline (15). Similar studies carried out by Sadri et al. and Moazami et al. (22, 23) reported high frequency of resistance to aminoglycosides and fluoroquinolones. Colistin is the only antimicrobial agent that retains high activity against P. aeruginosa (24).
When the administration of a β-lactam, aminoglycoside, or quinolone is ineffective, the polymyxins, particularly colistin, remain as the antimicrobial drugs of last option. Furthermore, resistance to colistin is infrequently observed in spite of a daily selective pressure in patients receiving colistin by inhalation (25). Sadri et al. (22) showed 9.1% colistin resistance amongst P. aeruginosa from Tehran, Iran. In this study, 2.5% of isolates were colistin resistant. With increasing administration of polymyxins, polymyxin resistant P. aeruginosa isolates have been reported from around the world (25).
Some studies showed that combination of colistin with an antipseudomonal agent such as, imipenem, piperacillin, aztreonam, ceftazidime, azlocillin, rifampin or ciprofloxacin was more effective than only colistin on MDR P. aeruginosa (26). Our results showed high level of resistance to cephalosporin, anti-pseudomonas penicillin and carbapenems. β-Lactamases, enzymes open β-lactam ring and make antibiotics inactivated. These enzymes are coded by different genes located on chromosomes or plasmids. These enzymes, which are mostly called extended spectrum β-lactamases (ESBL), in Ambler classification are divided to four groups, A to D (27). Various Ambler’s class D ESBLs, such as OXA-type ESBLs have been identified and encountered most commonly in P. aeruginosa (27). Our findings showed the OXA- group I as the most frequent and OXA-4 as the least frequent bla-OXA with 56% and 2% frequency, respectively.
The results of the study of genes OXA-10 compared with the results obtained in neighboring countries like Saudi (OXA-10 = 56%) and Turkey (OXA- 10 = 55%) is equal (28, 29), yet a study in 2013, indicated that the frequency of genes OXA-10 in Iraq’s 100% indicated high rates of antibiotic resistance to P. aeruginosa in this country. The frequency of genes OXA-1, OXA-group I, II and III reported in France were 26%, 5%, 4%, 4%, respectively, and were lower compared to the results obtained in this study (4). However, in Korea the frequency of genes OXA-group I, II, III and OXA-1 was reported as 34%, 6%, 16% and 11%, respectively. Some factors such as diversity of antibiotic use and geographic difference may effect diversity of antibiotic susceptibility (22).
The analysis of ERIC-PCR indicated clonal and high genetic diversity among P. aeruginosa isolates isolated from Tabriz hospitals compared with similar studies in Iran (18). This finding may be due to patients’ reception from around the province of eastern Azarbayjan in Tabriz; also it can be due to differences in the analysis or difference in software used in the analysis. These results show high frequency of antibiotic resistant P. aeruginosa in our hospitals. High genetic diversity, high frequency of OXA enzymes and MDR isolates emphasize requirement of an appropriate program to manage and control these isolates.