1. Background
According to the international society for human and animal mycology (ISHAM), onychomycosis is an invasive fungal infection of the nails, without regard to the causative agent (1). This disease may involve the toenails or fingernails, and represents approximately 30% of superficial mycoses (2). It is the most common disease of the nails, constituting approximately half of all nail abnormalities, with an increasing incidence with age (3). The causative agents of onychomycosis include dermatophytes, yeasts, and non-dermatophyte molds (NDMs) (4). The term tinea unguium is reserved for onychomycosis caused by dermatophytes; however, tinea unguium and onychomycosis are sometimes considered synonymous. In this article, the term onychomycosis refers to infections caused by either dermatophytes, yeasts, or NDMs.
The estimated prevalence of onychomycosis is more than 10% in the general population and 40% in elderly individuals, probably due to suboptimal immune function, inactivity, and the inability to maintain good foot care (5). It has been reported that the majority of onychomycoses are caused by dermatophytes, while yeasts and NDMs each account for approximately 10% of onychomycosis cases worldwide (3). Non-dermatophyte onychomycosis (NDO) is caused by hyaline (6, 7) and dematiaceous (8, 9) filamentous fungi that are commonly found as soil saprophytes or plant pathogens. Unlike dermatophytes, they are generally not keratinolytic (10). They live on the unkeratinized intercellular cement of the host tissue and must take advantage of previous keratin destruction by dermatophytes, trauma, or another nail disease. For this reason, they are sometimes considered secondary invaders of the nail plate (11). In Iran, a significant percentage of onychomycosis is caused by non-dermatophytes (12-14).
Although the list of NDM species that have occasionally been isolated from nails is quite long, only a few are regularly identified as real causes of onychomycosis. These include Scopulariopsis brevicaulis spp., Fusarium spp., Acremonium spp., Aspergillus spp., and Scytalidium spp. (15).
The epidemiological profiles of the causative agents of onychomycosis tend to alter over time, due to climatic, environmental, or socioeconomic factors, and can also be influenced by tourism (16). This variation may also reflect geographic differences in mold distribution, differences in the criteria used for diagnosis, and/or the use of mycological media for mold growth. Differentiation of the isolates is clinically important for targeted therapeutic decisions and for the prognosis, and for epidemiological purposes.
2. Objectives
The aim of this study was to determine the overall prevalence of dermatophytes, yeasts, and NDMs as the causative agents of suspected onychomycosis in patients in Tehran, Iran. This study is one of the large-scale reports done in a short period (one year) on the microbial epidemiology of onychomycosis in Iran.
3. Methods
3.1. Study Population
A total of 1,069 nail-clipping specimens were obtained from outpatients with suspected onychomycosis in Tehran during 2014 - 2015. The patients had been referred to three medical mycology laboratories for routine diagnostic procedures.
3.2. Mycological Examination
After cleaning the affected area with 70% ethanol, nail scrapings were collected from the deepest part of the nail and as close as possible to the intact parts of the nail by scraping the nail bed, the underside of the nail plate, and the hyponychium. One piece of each collected nail fragment was examined using a potassium hydroxide (KOH 20%) preparation to identify the presence of any fungal elements, including hyphae, arthrospores, yeast cells, and pseudohyphae. For a total of 788 samples, another part of the nail was cultured in plates containing Sabouraud dextrose agar (Difco, Detroit, MI, USA), with and without 0.05% cyclohexamide and 0.005% chloramphenicol, by inoculation of sample fragments onto the three points of an agar plate and incubating them at 28°C for 1 - 4 weeks. The cultures were checked twice weekly for evidence of growth. No growth at the fourth week was considered a negative culture. The criteria for a diagnosis of NDM onychomycosis was made based on nail abnormalities consistent with this diagnosis, a positive KOH preparation with the presence of specific hyphae in the nail keratin, and, when the culture was done, the failure to isolate a dermatophyte in the culture and growth of identical mold colonies in the inoculation sites of the culture media. Samples with characteristic saprophytic hyphal elements on direct microscopy and significant growth of NDMs on culture were considered for species identification by colony morphology and a microscopic examination with lactophenol cotton blue preparation according to identification keys (17).
3.3. Statistical Analysis
Demographic and microbiologic data were analyzed using the SPSS (version 21.0) statistical package (18).
4. Results
The study population comprised 643 females and 426 males. All nail specimens were subjected to direct microscopic examination, while cultures were also performed on 788 (73.7%) of the samples based on physicians’ requests. According to the microscopic tests, the prevalence of onychomycosis was 39.6% (n = 424), found in 185 males and 239 females. Fingernail onychomycosis was recognized in 38.3% of the cases, toenail onychomycosis in 59.1%, and a combination of both in 2.6%. Fingernail onychomycosis was significantly more prevalent in females than in males (120 versus 42), while toenail infections were significantly more common in males than in females (136 versus 115).
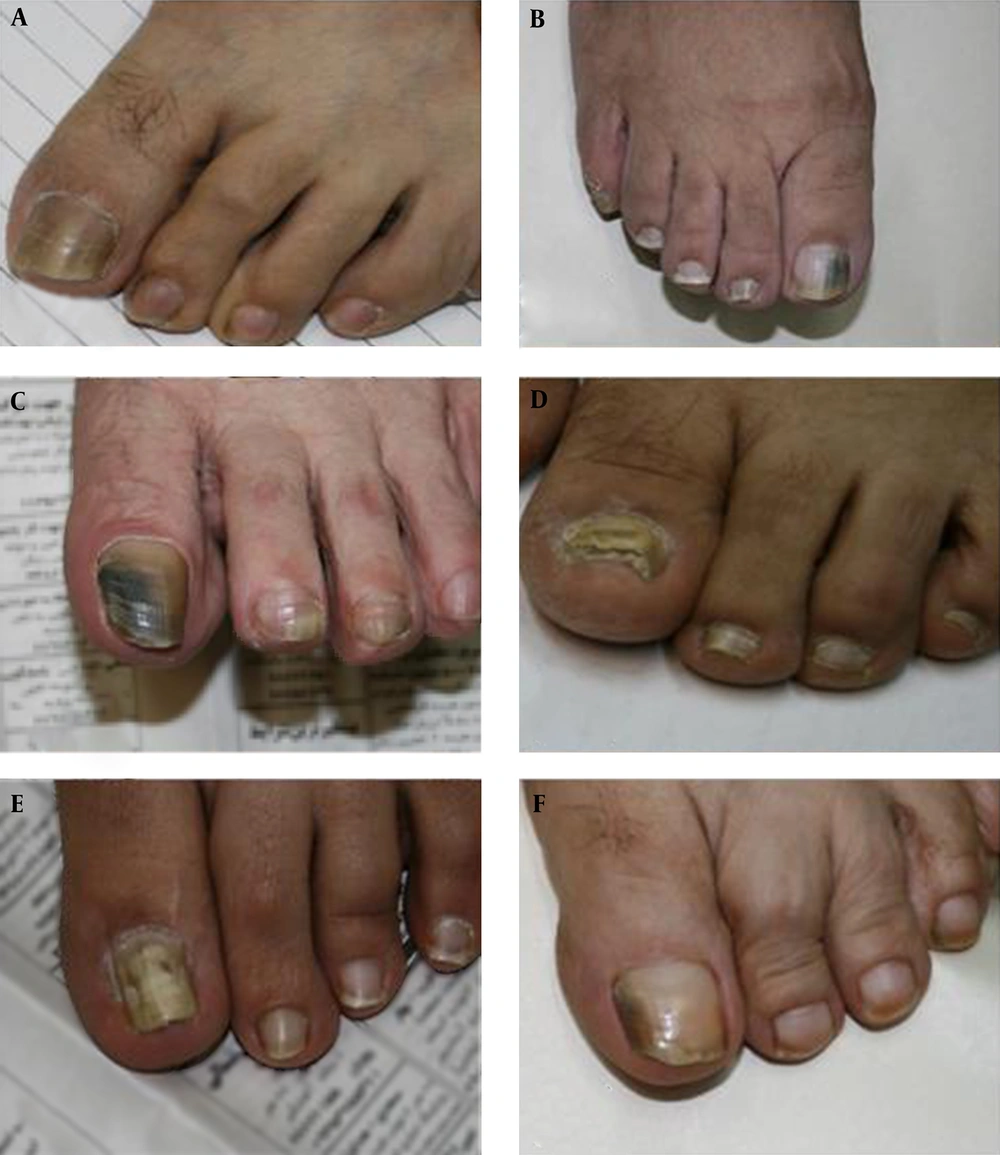
A total of 152 (35.8%) of the 1,069 samples showed the branching mycelium and/or arthroconidia representative of dermatophytes, 139 (32.7%) showed the blastoconidia and pseudohyphae representative of yeasts, and 124 (29.3%) showed the saprophytic mycelia representative of NDMs. Mixed infections were observed in 2.2% (n = 9) of the positive cases (Table 1). Various clinical forms of onychomycosis due to NDMs were seen among the samples; examples are shown in Figure 1. The causative factors for fingernail onychomycosis were Candida spp. (66.2%), dermatophytes (10.4%), NDMs (21%), and mixed infections (2.4%). The rates of the same factors for toenail onychomycosis were 10%, 52.8%, 35.2%, and 2%, respectively.
The results indicated that laboratory confirmation was achieved through direct examination with cultures in 726 samples, and by only a positive direct exam in 45 cases or only a positive culture in 17 cases (Table 2). The most commonly isolated NDO was Aspergillus spp. (69.3%, n = 52), followed by Fusarium spp. (n = 7). There were fewer isolated Paecilomyces spp., Scopulariopsis spp., Acremonium spp., Cladosporium spp., and Chrysosporium spp., each with only one case (1.3%). The causative agents in 11 of 75 cases of NDM onychomycosis were not diagnosed with culture colonies.
| Dermatophyte (Finger/Toe) | Candida (Finger/Toe) | Mold (Finger/Toe) | Mix (Finger/Toe) | Negative (Finger/Toe) | Total (Finger/Toe) | |
|---|---|---|---|---|---|---|
| By direct examination | 152 | 139 | 124 | 9 | 645 | 1,069 |
| By culture | 94 | 101 | 75 | 12 | 506 | 788 |
| Culture-Positive | Culture-Negative | Total | |
|---|---|---|---|
| Microscopy-positive | 265 (33.6) | 45 (5.7) | 310 (39.3) |
| Microscopy-negative | 17 (2.2) | 461 (58.5) | 478 (60.7) |
| Total | 282 (35.8) | 506 (64.2) | 788 (100) |
aValues are expressed as No. (%).
5. Discussion
Correctly determining the etiologic agents of onychomycosis is important in order to provide a baseline for administering appropriate antifungal therapy and identifying the source of infection, hence facilitating prevention measures. An inaccurate clinical diagnosis may prolong the patient’s discomfort and result in a financial burden due to expensive antifungal therapy (19).
In the present study, the incidence of onychomycosis was confirmed in 39.6% of the examined patients. Although a higher prevalence of onychomycosis was reported in other studies conducted in different regions of Iran, such as Sari (56.8%) (14), Khoozestan (42.9%) (20), and Kermanshah (45.2%) (13), the incidence in our study was more than in some older Iranian studies, such as those by Asadi et al. (18.9%) (21) and Moghaddami et al. (28.9%) (22). In our samples, onychomycosis affected toenails (59.1%) more often than fingernails (38.3%), probably due to toenails’ slow growth, which facilitates the invasion of the fungus and is perhaps supported by factors such as trauma and poor circulation (23). Also, onychomycosis affected more females (56.4%) than males (43.6%) in the present study. A higher incidence of onychomycosis in women has been reported in other studies (24-26).
The etiological fungal agents were dermatophytes (35.8%), yeasts (32.7%), NDMs (29.3%), and mixed infections (2.2%) in the present study. Other local studies conducted in the cities of Ghazvin (26) and Tehran (27) showed that dermatophytes were the major causative pathogens. Similarly, in studies performed in Mexico and Malaysia, dermatophytes were the principal pathogens (28, 29). Nevertheless, the epidemiology and etiology of onychomycosis varies in different geographic areas, as summarized in Table 3.
| Year | City | Total Samples (n) | Prevalence of Onychomycosis (%) | Prevalence of NDMs (%) | Prevalence of Dermatophytes (%) | Prevalence of Candida spp. (%) | Commonest NDM spp. | Other isolated NDMs | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1989 | Tehran | 927 | 28.9 | 1.86 | 32.1 | 66.04 | Aspergillus spp. | Penicillium spp. | (22) |
| 2000 | Khoozestan | 2,525 | 42.9 | 2.1 | 10.4 | 87.5 | A. fumigates | A. niger, Scopulariopsis brevicaulis, Fusarium spp., A. flavus, Alternaria spp. A. terreus, Mucor spp. | (20) |
| 2001 | Tehran | 115 | 84.3 | 8.2 | 48.4 | 43.3 | S. brevicaulis | Aspergillus spp., Acremonium spp., F. solani | (27) |
| 2002 | Tehran | 252 | 39.9 | 14.2 | 31.8 | 54 | A. flavus | A. niger, Scopulariopsis brevicaulis, Fusarium spp. | (30) |
| 2009 | Kashan | 137 | 18.9 | 23 | 34.7 | 42.3 | A. flavus | A. fumigatus, Scopulariopsis spp., Fusarium spp. | (21) |
| 2009 | Tehran | 549 | 47.9 | 32.6 | 21.9 | 45.5 | A. flavus | A. fumigates, A. niger, Penicillium spp., Rhizopus spp., Cladosporium spp., Acremonium spp. | (25) |
| 2010 | Esfahan | 488 | 39.8 | 28.4 | 13.9 | 57.7 | A. flavus | A. nidulans, A. fumigates, Acremonium spp., Cladosporium spp., Scopulariopsis brevicaulis, Fusarium spp., Penicillium spp. | (24) |
| 2010 | Tehran | 504 | 42.8 | 19 | 21.3 | 59.7 | A. flavus | Aspergillus spp., Fusarium spp., Penicillium spp., Scopulariopsis spp. | (12) |
| 2010 | Qazvin | 308 | 40.2 | 3.2 | 50.2 | 46.6 | A. niger | A. flavus | (26) |
| 2013 | Kermanshah | 1,086 | 45.2 | 2.9 | 18.6 | 78.5 | A. flavus | Aspergillus spp. | (13) |
| 2014 | Sari | 1,100 | 56.8 | 15 | 23 | 62 | A. flavus | A. fumigatus, Fusarium spp., Scopulariopsis brevicaulis, Geotrichum spp., Trichosporon spp., Cladosporium spp.,Penicillium spp. | (14) |
Unlike many studies performed in Iran (13, 14, 20), in the present survey, the frequency of onychomycosis caused by NDMs was almost equal to the frequency of nail infections caused by dermatophytes and yeasts. Among the studies done in Tehran, the frequency difference between the most common causes of onychomycosis (dermatophytes or yeasts) and NDMs was 35% - 64% (12, 22, 27, 30). This difference was only 6% in our study, which is similar to a previous study carried out in Tehran in 2009 (25).
The prevalence of NDMs isolated from nail infections in various parts of the world ranges between 1.49% and 33.5% (30-33); however, it seems that this rate has increased dramatically in the past several years (34, 35). Although our study is not a comprehensive epidemiological survey and we did not test all samples, these random data demonstrate an increasing occurrence of onychomycosis due to NDMs. This study demonstrated that 29.3% of unusual onychomycosis cases are due to NDMs, which is 1.5 times more than the 19% found in the last study conducted in Tehran (2010) (12).
The increased incidence of NDOs may be due to the widespread use of broad-spectrum antibiotics and the increased frequency of immunosuppression, chemotherapy, debilitating diseases, metabolic diseases such as diabetes, occupational accidents, aging of the population, and any other factors that predispose the nails to the invasion of pathogens. Thus, NDMs should be considered important pathogens, with a high index of suspicion in evaluating patients with cultures that are negative for dermatophytes, or in those experiencing treatment failure (10). Non-dermatophyte onychomycosis presents clinicians with a greater diagnostic challenge compared to dermatophyte onychomycosis. The latter can be diagnosed with the single isolation of a dermatophyte, but NDM onychomycosis requires further measures for confirmation (36).
The prevalence of the fungi responsible for NDOs varies considerably in different studies reported in the literature. In general, the top five organisms in terms of published confirmed isolates worldwide are Scopulariopsis brevicaulis, Fusarium spp., Aspergillus spp., Scytalidium dimidiatum, and Acremonium spp. According to the data in the present survey, the overall prevalence of NDM onychomycosis due to Aspergillus spp. is 69.3%. Although a study conducted in Tehran in 2001 (27) reported that Scopulariopsis brevicaulis spp. were the most common agent of NDO, other studies carried out in different areas of the country (13, 14, 37), including Tehran (12, 22), revealed that a large percentage of NDMs are Aspergillus spp., particularly A. flavus. In recent years, onychomycosis caused by different Aspergillus species has increased, as evidenced by case reports and epidemiological studies (38, 39). It is noteworthy that there was a case of a nail infection by Chrysosporium among our samples, which is the first confirmed case of onychomycosis caused by this species in Iran.
The prevalence found in this survey of onychomycosis due to NDMs was higher in toenails (77.7%) than in fingernails (22.3%), which is similar to results reported by Nouripour et al. (70.3%) (40), Khosravi et al. (87.5%) (27), and Zaini et al. (80%) (25). In contrast to our observation, an epidemiological survey in Khoozestan, southwest Iran, noted that NDMs were higher in fingernails than in toenails (20).
In conclusion, NDO appears to be an increasing problem in Iran, with a growing trend of NDMs isolated from onychomycosis compared to other causative agents of onychomycosis, noticeable in our samples and in other recent studies in Tehran. Since the published data on NDMs are limited, more studies on this group of fungi are recommended to clarify aspects of its epidemiology and pathogenesis.