1. Background
Cervical cancer is one of the most common types of cancer among women. The annual incidence and mortality rates of this disease were reported to be over 500,000 and 250,000 cases, respectively, with incidence rate of 85% in the developing countries (1-4). The risk of cervical cancer has increased along with the incidence of high-risk types of human papillomavirus (HPV). Therefore, these genotypes are considered as a significant risk factor for the development of cervical cancer. In fact, nearly all cases of cervical cancer are caused by infection with high-risk types of HPV (5-9). Human papillomavirus is a relatively small, nonenveloped, double-stranded DNA genome that is approximately 8000 base pairs in size (10, 11). This virus can infect cutaneous and mucosal epithelial tissues of the anogenital tract, hands, or feet and may give rise to a wide variety of diseases, ranging from cutaneous and anogenital warts to laryngeal papillomatosis and anogenital intraepithelial neoplasia, which often progress to malignancy (12).
To date, over 150 different types of HPV have been fully identified, approximately 40 of which are known to infect epithelial cells in the anogenital region (13). Human papillomavirus types infecting the genital tract can be divided into high-risk and low-risk groups, depending on their potential to cause cancer (13, 14). Low-risk HPV types (such as HPV6 and HPV11) commonly cause benign warts and low-grade premalignant lesions, which regress and do not progress to cancer. On the other hand, high-risk HPV types are associated with the development of anogenital cancer (12, 14). Certain strains of HPV are associated with different risk levels of transformation into genital tract in both males and females (13, 15-17). High-risk HPV16 and HPV18, which are found in high frequencies in cervical cancer, account for approximately two-thirds of all cervical carcinomas worldwide (HPV16 as the most frequent type) (16-22).
The presence of even minimal amounts of HPV DNA is associated with an increased risk of cervical cancer development (23). Human papillomavirus DNA is detected in the majority of cervical tumours (12, 14, 24), while its frequency rate is typically below 10% in the cervical tissues of healthy women (25). Generally, persistent infection of cervical epithelium with high-risk HPV types is necessary, but it is not a sufficient etiological factor for the carcinogenesis of cervical cancer (26). In addition, the majority of positive biopsies were shown to contain several additional HPV types.
Although HPV infection is very common, most infections are suppressed by the immune system within one to two years without causing cancer. However, the mechanisms by which the cellular immune response eradicates HPV infection are not yet clearly understood. Some of these lesions are not removed by the immune system and progress into cancer. In fact, it can take several decades for a persistent infection with high-risk HPV to develop into cancer (26-28). Therefore, it is important to determine the epidemiology of HPV genotypes in normal tissues, as well.
2. Objectives
This study was carried out to determine and analyze the distribution of HPV genotypes in normal cervical tissues, using polymerase chain reaction (PCR) in Mazandaran province, situated in North of Iran, during 2012 - 2015.
3. Methods
3.1. Samples
This cross-sectional study was performed during 2012 - 2015, tissue samples from the cervix were collected from 450 women who were referred to a healthcare centre in Mazandaran province. Information such as age, gender, and suspected source of infection (e.g., high-risk sexual intercourse and smoking) was collected from all the patients using a questionnaire. This study was approved by the ethics committee of Mazandaran University of Medical Sciences, Sari, Iran, and written informed consent was obtained from all the patients’ participating in the study.
3.2. Pap Test
Cervical cytology screening or Pap smear is a test used to detect abnormal cells from the vagina and cervix. Pap test is a simple method that can easily detect cancerous or precancerous conditions. In this test, cells from the cervical canal are collected; Pap smear is also used to monitor any abnormalities or unusual findings and can detect cervical cancer in the early stages. In the present study, the samples were collected using a plastic spatula, and the cells were scraped off from the cervical region (a completely painless procedure). The specimens were collected and placed in a special liquid preservative. The cell suspension was dropped into a glass slide, stained, and then examined under a microscope by a cytopathologist.
3.3. DNA Extraction
The scrapings from cervical tissue samples were placed in sterile microtubes. DNA extraction was performed using a DNA isolation kit (Roche Diagnostics Deutschland GmbH, Mannheim, Germany) according to the manufacturer’s instructions. Briefly, 200 µL of tissue lysis buffer (4 M urea, 200 mM Tris, 20 mM NaCl, and 200 mM EDTA; pH = 7.4) and 40 µL of proteinase K were mixed and incubated overnight at 37°C. On the following day, 200 μL of binding buffer (6 M guanidine-HCl, 10 mM urea, 10 mM Tris-HCl, and 20% triton X-100 [v/v]; pH = 4.4, 25°C) was added to the tube and vortexed. To elute DNA, 200 µL of pre-warmed elution buffer (70°C) was added. DNA extracts were stored at -20°C prior to use. The DNA quality and absence of PCR inhibitors in the samples were tested through PCR with β-actin primer as a housekeeping gene. To monitor contamination during PCR, distilled water was employed as negative control.
3.4. HPV DNA Detection and Genotyping
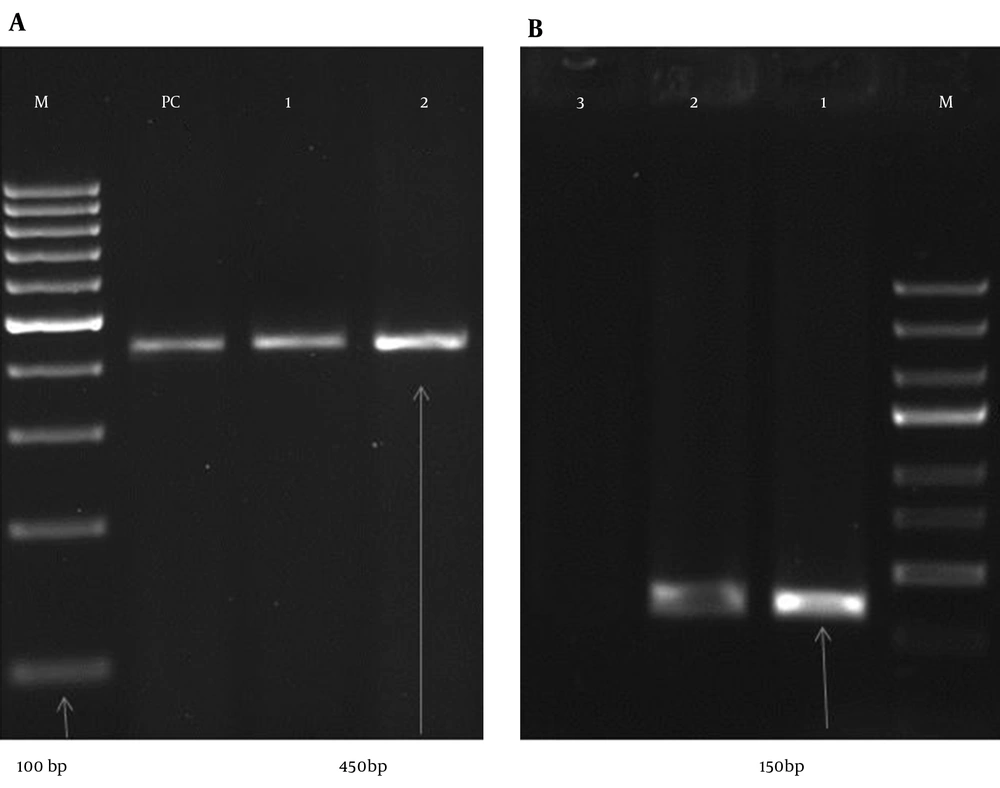
GP5 + / GP6 + primer was applied as the general primer to amplify the L1 gene (Table 1). PCR was performed, using 1X PCR buffer (Invitrogen, USA), 3 mM MgCl2 (Invitrogen, USA), 0.2 mM dNTPs, 0.5 U Taq DNA polymerase (Invitrogen, USA), 25 pmol of each primer, 500 ng of genomic DNA, and sterile distilled water to the final volume of 25 µL. Amplification was performed with cycles of 5 min at 94°C for primary denaturation, followed by 40 cycles at 94°C (1 minute), 48°C (60 seconds), and 72°C (60 seconds), with a final 10 min extension step at 72°C. HeLa cell line (HPV18 positive) was used as the HPV positive control. Finally, 10 µL of each PCR product was analyzed by 1.5% agarose gel electrophoresis. To determine the HPV genotype in the positive samples, we used HPV HCR genotype-EPh PCR kit (Amplisens®, France). Human papillomavirus-positive and -negative samples were used for every sample run.
| Primers | Sequences 5 - 3 |
|---|---|
| GP5+ | TTTGTTACTGTGGTAGATACTAC |
| GP6+ | GAAAAATAAACTGTAAATCATATT |
| β-actin (F) | CTGCCGTTTTGCGTAGGAC |
| β-actin (R) | AGGCGTACAGGGATAGCAC |
3.5. Gel Electrophoresis
To determine HPV genotypes, PCR products were examined by 1.5% agarose gel electrophoresis. Since all the amplified products had different lengths, the virus genotypes were analyzed by electrophoresis and visualized by an ultraviolet transilluminator. Bands of an appropriate size were identified through comparison with DNA molecular weight markers, which are a set of known DNA fragments. The adequacy of DNA in each specimen for PCR amplification was determined by the detection of β-actin gene.
Chi-square or Fisher’s exact test were performed, using SPSS version 14, to determine the relationship between the presence of HPV DNA and smoking habits, alcohol drinking, weight loss, family history of cancer, anatomical site of tumor, pathological diagnosis, and metastasis.
4. Results
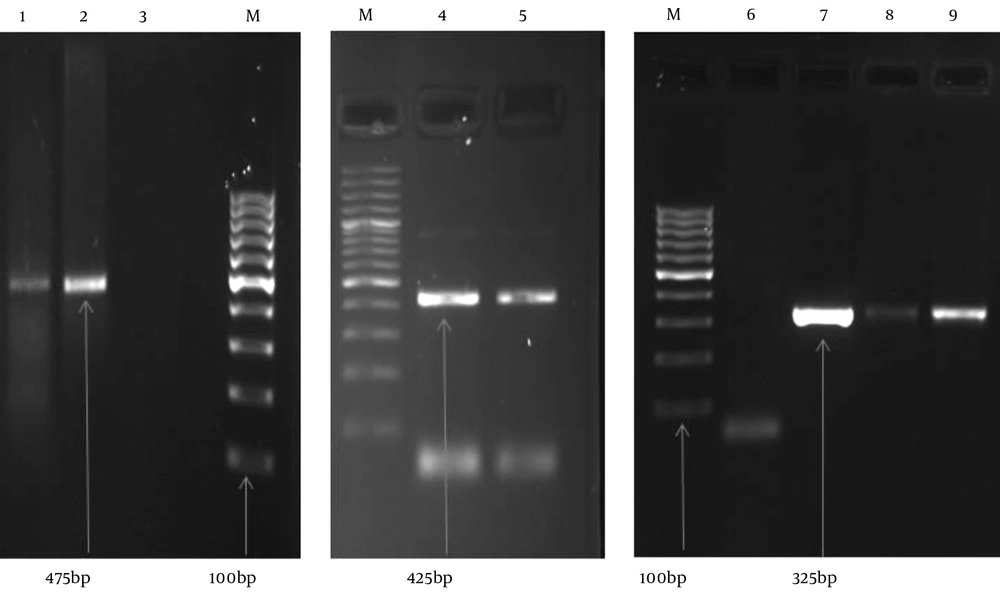
In the current study, 450 cervical samples were collected using a plastic spatula from women referring to the healthcare centre in Sari, situated in North of Iran, during 2012 - 2015. In this study, 431 (95.78%) of the cytological samples were found to be normal on Pap smear test, while 19 (4.22%) of the cytological tissue samples were abnormal. First, HPV DNA was detected by PCR, using GP5 + / GP6 + primers. Second, HPV genotyping was identified using the HPV HCR genotype-EPh PCR kit (Amplisens, France). Based on the obtained results, HPV DNA was detected in 46 (10.22%) of the cytological samples from the cervix. From the 46 HPV DNA positive samples, 19 (41.30%) were HPV16, 9 (19.57%) were HPV18, and 1 (2.17%) was HPV45 (Table 2).
| Genotypes of HPV | High Risk HPV with Normal Cytology (%) | High Risk HPV with Abnormal Cytology (%) | Total High Risk HPV (%) |
|---|---|---|---|
| HPV16 | 13 (44.83) | 6 (20.70) | 19 (65.52) |
| HPV18 | 6 (20.70) | 3 (10.34) | 9 (31.04) |
| HPV45 | 1 (3.45) | 0 (0) | 1 (3.45) |
| Total | 20 (60.97) | 9 (31.04) | 29 (100) |
In this study, the highest number of cervical samples was collected from women aged 25 - 29 years (19.56%), and HPV DNA was detected in 15.91% of cervical samples in this age group. Nevertheless, the highest prevalence of HPV DNA was detected in the subjects aged 20 - 24 years (28.17%), and HPV DNA was collected from 4.44% of the cervical samples from women aged 20 - 24 years. On the other hand, a lower prevalence of HPV DNA was reported in the cervical samples from subjects aged 15 - 19 or > 40 years (Table 3). Human papillomavirus DNA was detected in 8.12% of normal tissues (35 HPV-positive samples in 431 studied women) and 57.89% of abnormal tissues (11 HPV-positive samples in 19 studied women). Among normal cytological samples, 20 (4.64%) were identified as high-risk HPV, whereas in the abnormal cytological samples, 9 (47.39%) of the samples were identified as high risk HPV. Our results showed that the rate of positive HPV DNA was 6.32% in non-smokers (27 in 424 non-smoker samples) and 73.08% among smokers (19 in 26 smoker samples). The gel electrophoresis patterns of HPV DNA and high-risk HPV types are presented in Figures 1 and 2, respectively.
| Age (years) | Number of Samples (%) | HPV+ in Groups (%) | HPV+ in Total (%) |
|---|---|---|---|
| 15 - 19 | 4 (0.89) | 0 (0) | (0) |
| 20 - 24 | 71 (15.78) | 20 (28.17) | (4.44) |
| 25 - 29 | 88 (19.56) | 14 (15.91) | (3.11) |
| 30 - 34 | 69 (15.33) | 3 (4.35) | (0.67) |
| 35 - 39 | 81 (18) | 5 (6.17) | (1.11) |
| 40 - 44 | 72 (16) | 2 (2.78) | (0.44) |
| 45 - 49 | 65 (14.44) | 2 (3.08) | (0.44) |
| Total | 450 (100) | 46 (10.22) | (10.22) |
5. Discussion
Clinical and epidemiologic evidence exhibited a strong relationship between cervical cancer and HPV infection and a high frequency of oncogenic HPV DNA in abnormal cervical tissue. Infection by certain HPV types is recognised as a causal and necessary factor for the development of cervical cancer, and HPV DNA may be detected in virtually all cervical cancer patients (3, 29). Histopathological results of 450 cervical samples of our study, revealed that 431 (95.78%) of specimens were normal while 19 (4.22%) of them were abnormal. It was reported that the prevalence rate of the abnormal cervical cytology was 11.6% (30), which is more than to our results. Another study revealed a prevalence rate of 1.8% for cervical cytological abnormalities (31), which is lower than to our results. It was reported that 95.07% of the samples were normal tissue and 4.93% were abnormal cytology (32), which is similar to our results. Human papillomavirus on these samples demonstrated that HPV DNA was found in 46 (10.22%) of cervical specimens. This rate was much higher in Sub-Saharan African regions (24%) (33), Turkey (23% and 20%) (30, 34), Latin America (16.1%), Eastern Europe (14.2%), and Southeast Asia (14%).
In line with our study, the prevalence of HPV types in women with normal cervical cytology was reported to be 10.4% (35). On the other hand, a study performed in North America indicated a much lower HPV prevalence (4.7%) (36). In this study, HPV DNA was detected in 8.12% (35 out of 431 normal samples) and 57.89% (11 positive HPV DNA samples out of 19 abnormal samples) of cytological tissue samples. The reason for the high prevalence of HPV might be the method applied for HPV detection.
In the literature, the prevalence of HPV increased as the date of study publication progressed; this is in fact due to the technological advances in HPV detection methods (37-40). We found HPV16 in 19 (41.30%), HPV18 in 9 (19.57%), and HPV45 in 1 (2.17%) of HPV positive samples. Human papillomavirus16 and HPV18 had the highest frequencies in cervical cancer (22, 41), which is similar to our result. Another result showed that the frequencies of HPV35 and HPV31 were the highest (32), which is inconsistent with our result. In a previous study, the prevalence of HPV types, including both low-risk and high-risk types, was estimated at 25.7%, while high-risk HPV was detected in 23.0% of cases (30), which is more than twice as high as the present rate. In the present study, the highest prevalence rates of HPV DNA were found in the age groups of 20 - 24 years (28.17%) and 25 - 29 years (19.56%) (30), which is similar to a previous study. In a study performed in South of Iran on 799 participants, 26.3% were aged between 17 and 30 years, 36.5% between 31 and 40 years, and 37.2% between 41 and 50 years. In that study, the prevalence of HPV increased with age (42), which is not similar to our result.
In the abovementioned study, the prevalence of all HPV types (including high-risk HPV) was higher in women younger than 30 years, compared to women aged 30 or above. Moreover, the highest prevalence of infection was found in women aged 20 - 29 years. In this age group, the prevalence of HPV was estimated at 31.2% and the prevalence of high-risk HPV was 25.9% (30); overall, these findings are in congruence with the present results. Generally, the available data on the prevalence of cervical HPV infection is considerably limited in the Iranian population.
In the present study, the rate of positive HPV DNA was 6.34% in non-smokers and 73.08% in smokers; in fact, the prevalence of HPV was higher among smokers in comparison with non-smokers. The present findings were consistent with those of the previous studies, confirming that smoking is a risk factor for HPV infection (43, 44). Certain types of HPV are known to be associated with cancer. Cervical cancer screening, comprising of both Pap test and HPV testing, is an essential part of the routine healthcare for women, as it can detect abnormal cervical cells that may cause cervical cancer. Therefore, it is important to detect abnormal cervical tissues before they cause cervical cancer.