1. Background
Hepatitis E virus (HEV) [belongs to the Hepeviridae family and genus Hepevirus] is a causative agent of the hepatitis E infection with a mortality rate of about 0.5% to as high as 25% in pregnant women and immunosuppression individuals especially in high endemicity regions of the world (1). The clinical manifestation of the disease varies from subclinical to severe hepatitis, which may result in liver failure with consequences of morbidity and mortality (1, 2). Hepatitis E virus is a water-borne infection and oral-fecal is the main route of transmission due to drinking water contamination (3). Additionally, HEV transmission has been reported via blood transfusion and food contamination derived from infected animal products (4). Based on current seroepidemiological and molecular data, the endemicity of HEV has been defined as high endemic regions comprising South and East Asia, Central Asia, Middle East and Africa.
The epidemic cases are in South and Southwest Asia, North Africa and the Middle East (2, 4). Manifestations of the HEV infection in endemic regions are epidemic and sporadic hepatitis (4). The Hepatitis E virus is a non-enveloped RNA positive sense about 7.2 kilobases. Hepatitis E virus comprises three open reading frames among with Open Reading Frame 2 (ORF2) contains 660 amino acids (aa) encodes the capsid protein (3). The capsid encompasses S, M, and P domains. P or E2S domain is responsible for host interaction, specific immune response and genotyping determination (1). Based on nucleotide sequences of ORF1, ORF2 and ORF3 regions, HEV has been classified into 5 genotypes among which genotypes 1 and 2 are reported only in humans, genotypes 3 and 4 are zoonotic infections and genotype 5 is restricted to animals. All these HEV genotypes share one serotype (4).
With regards to consequences and lack of specific treatment, the immunization can be an effective option for prevention of the HEV infection. Since no cell culture system has been adopted for cultivation of HEV, the preparation of vaccine remains a great challenge. So far, several efforts have been made to develop HEV vaccines through expression of different truncated ORF2 in many expression systems such as: Escherichia coli, yeast, plant, mammalian and insect cells (1, 5-7). The BES is an efficient insect system for HEV vaccine development because of its capacity to produce a 56 KDa protein from amino acid 112 to 607 (8). The neutralizing epitope of this protein is between 457 and 607 region (5). The truncated 112 - 578aa and 112 - 660aa of the ORF2 HEV have also been investigated in the BES (5, 7).
It is well established that adjuvants play an important role in inducing long-term immunity and enhancing the efficacy of vaccines (9). Several adjuvants have been used in the vaccines development and amongst them aluminum hydroxide, aluminum phosphate and MF59 are widely used (10, 11). Recently, the HEV vaccine that is produced in BES used Aluminum hydroxide (alum) as an adjuvant (12). However, adjuvants with more ability to elicit the mucosal immunization are now interested. It is believed that the mucosal adjuvants in addition to induction protective immunity against infectious diseases are possibly able to treat the immunological diseases (13). Additionally, some putative proteins were used as an adjuvant and were found to boost mucosal immunity (12).
It has been shown that mucosal immunization with mutant forms of Cholera (mCT) and heat-labile toxin (mLT) without toxicity retaining adjuvanticity properties (14). Moreover, it has been reported that the full-length and cleaved product (112 - 175) of simian SA11C13, full length NSP4 of virulent and attenuated porcine Rotavirus strains (OSU-v and OSU-a) have highly adjuvant activity without toxicity (14). NSP4 is a nonstructural protein that encodes by gene 10 of the Rotavirus that contains 175aa with enterotoxin activity at position 114 to 135. This protein contains different domains with multifunctional activity such as morphogenesis, pathogenesis and induction of the immune response, which has been incorporated in the Rotavirus vaccine (14, 15).
2. Objectives
This study was aimed to design and construct the truncated form of the HEV ORF2 protein with deletion at 111aa from N-terminal and 52aa from C-terminal in the baculovirus expression system. Additionally, the main goal of this study was to link the mutant form of the NSP4 protein (OSU-a strain) of Rotavirus in C-terminal of truncated ORF2 protein and assesses the expression of the truncated ORF2-NSP4 combination. Obtaining this recombinant protein can be a new potential candidate vaccine against HEV and Rotavirus infections.
3. Methods
3.1. Gene Synthesis
NSP4 (OSU-a strain), and HEV genotype 1 ORF2 complete nucleotide sequences were retrieved from the NCBI gene bank (accession number: D8883). Then NSP4 was linked to C-terminal of truncated ORF2 (112 - 607) via specific linker (glycine-glycine-glycine-serine) using the Vector NTI software (Vector NTI Advanced™ 11).
Two restriction enzyme digestion sites including BamHI at N-terminal and SalI at C-terminal were designed in the fusion truncated ORF2-NSP4 gene. To confirm our digestion, the virtual digestion was performed using vector NTI. MEGA software version 5.0 (Biodesign Institute, Tempe, AZ, USA) was used for alignment of the constructed sequence with the ORF2 gene. The designed gene was codon optimized in the baculovirus based expression system, synthetized and cloned in to pBlue Script II SK (+) cloning vector (Biomatik company, Canada) (http://www.biomatik.com).
3.2. Cloning of Truncated ORF2 (112-607)-NSP4 in to the PfastBac1 Plasmid
The truncated ORF2 gene was separated from PBlueScript II SK (+) and was amplified using the following primers: ORF2 Forward: 5-CTCGAGGGATCCA TGGCC-3 and ORF2 Reverse: 5-ACGCGTCGAC GGCCAGCACGGAGTGTGGA-3. The ORF2 forward and reverse primers contain BamHI and SalI restriction sites (underlined), respectively. Then the truncated ORF2 gene was inserted into the linearized pfast Back 1 and then transformed in E.coli DH5α. Then the selected colonies cultured on Luria Bertani medium (LB) (Merck, Germany) agar plate containing ampicillin (50 mg/L).
The pBlue Script II containing fusion truncated ORF2-NSP4 was transformed in E.coli DH5α, cultured in LB agar containing ampicillin (50 mg/L), then plasmid was extracted and double digested by BamHI and SalI restriction enzymes (Thermo Scientific, USA). Simultaneously, PfastBac1 plasmid was also double digested by BamHI and Sal1. To confirm digestion, the products were run on the agarose gel. Then, truncated ORF2-NSP4 and linearized PfastBac1 were extracted by using the agarose gel DNA extraction kit (Roche, Germany) and ligated using the T4 DNA ligase (Roche, Germany). The recombinant PfastBac1 + truncated ORF2-NSP4 was transformed in to E.coli DH5α competent cells using CaCl2 and colonies were selected on LB agar plate containing ampicillin (50 mg/L).
3.3. Confirmation of Subcloning
The cloning sites of two cloned genes in the PfastBac1 plasmid was confirmed by single and double digestion, PCR and sequencing. Sequencing was carried out by the Bioneer Corporation (Daejeon, South Korea).
3.4. Transformation and Transposition of Pfastbac1 in to DH10Bac
In order for construction of recombinant Bacmid containing helper plasmid, the truncated ORF2-NSP4 and truncated ORF2 genes were retransformed to DH10Bac competent cells and white colonies selected in LB agar plate containing [in µg/mL] kanamycin 50, gentamycin 7, tetracycline 10, X-gal 100and IPTG40 (Bac-to-Bac®Baculovirus Expression System) and then colony PCR was carried out to confirm the transformation of target genes into Bacmid. The next step confirmed colonies purified with endofree mega kit (Qiagen, Germany). In order to reconfirmation the extracted recombinant Bacmid DNA, the following PCR condition was carried out according to the manufacturer’s instruction (Bac-to-Bac®Baculovirus Expression System) (Invitrogen, Germany).
The PCR reactions were performed in final volumes of 50 µL containing 1 μL Bacmid DNA (100 ng), 5 µL PCR reaction buffer (10X), 2.5µL of each primers (1.25 µL each 10 µM stock), 0.5µL of DNA platinum Taq polymerase (0.5 units/µL), 1.5 µL of MgCl2 (50 mM) and 1 µL of dNTP (10 mM). The thermal cycling conditions for the amplification was: initial denaturation at 94°C for 5 minutes followed by 35 cycles of 45s at 94°C, 45 seconds at 55°C and 5 minutes at 72°C. A final elongation cycle at 72°C for 7 minutes was also performed. The PCR products were analyzed on a 1% agarose gel. The sequences of pUC/M13 primers (Bioneer, Daejeon, South Korea) for amplification of Bacmid DNA were as follows: F (5-CCCAGTCACGACGTTGTAAAACG-3) and R (5-AGCGGATAACAATTTCACACAGG-3).
3.5. Transfection in to SF9 Cells
SF9 cells (Spodopterafrugipedra) derived from insect cells were purchased from the Razi Institute cell bank (Karaj, I.R.Iran). The cells incubated at 26.5°C in Grace medium, 10% Fetal Bovine Serum and 2mM non-essential amino acid (Gibco, USA). Then, the cells were transfected with pFastBac1-ORF2-NSP4Bacmid(OD:204ng/µL) and pFastBac1-ORF2Bacmid (700ng/µL) by polyfect-mediated method (Qiagen, Germany). The cells were incubated at 26.5°C with 8% Fetal Bovine Serum for 72 hrs. The cells again were infected with recombinant baculoviruses at a multiplicity of infection (MOI) 10. Following seven days of incubation at 26.5°C the cells were removed from culture medium. Finally, to evaluate protein expression the supernatant was collected and the cells were lysated with RIPA buffer (25 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate and 0.1% SDS, pH 7.6) containing protease inhibitor cocktail (complete mini, Roche, Indianapolis, IN, USA). The proteins were collected separately. The collected proteins were analyzed on SDS-PAGE.
3.6. Determination of Protein Expression by Western Blot
The expressed proteins, truncated ORF2 and truncated ORF2-NSP4 were separated by SDS-PAGE on 12% acrylamide gels and transferred onto a PVDF membrane. The membranes were blocked with 5% non-fat dry milk dissolved in Tris-buffered saline with 0.1% Tween 20 (TBST, pH 7.6) for 18 hrs. and then incubated overnight at 4°C with anti-ORF2 antibody (rabbit polyclonal, dilution 1: 250; Biorbyte; England). After four washes with TBST, membranes were incubated with a rabbit polyclonal secondary antibody to mouse IgG HRP; dilution 1: 1000) for 1.5 hr. at room temperature. Labeled proteins were detected using a chemiluminescence western blotting system.
4. Results
4.1. Subcloning the Truncated ORF2-NSP4 in pFastBac1
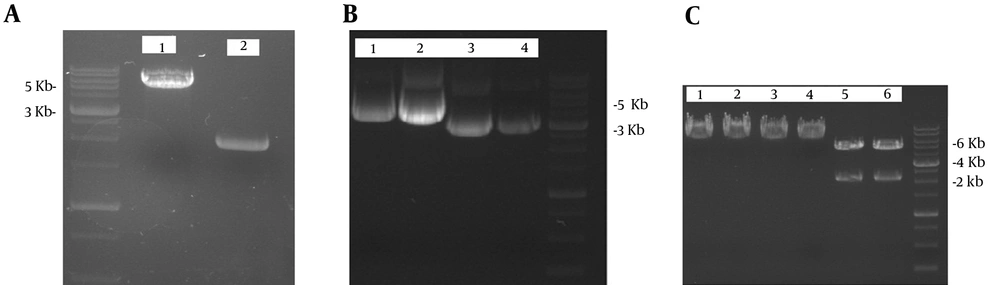
The purified double digested ORF2-NSP4 gene (2000 bp) was inserted into the pFastBac1 with cohesive ends. The position site of ORF2-NSP4 was confirmed by single and double digestion and the length of subcloned gene was 6775 bp (Figure 1).
A: The linearized pFastBac1 and ORF2-NSP4 respectively appeared at 4775 and 2000 bp. Lane 1: pFastBac1 is double digested with BamHI/SalI; Lane 2: pBlueScript-ORF2-NSP4 is double digested with BamHI/SalI.B: ORF2-NSP4 gene was cloned in pFastBac1. Lane 1 - 2: pFastBac1-ORF2-NSP4; and Lane3 - 4: pFastBac1. C: the position site of ORF2-NSP4 was confirmed by single and double digestion and the length of subcloned genes after single digestion was 6775 bp while after double digestion was 4775 and 2000 bp. Lane 1 - 2: pFastBac1-ORF2-NSP4 single digestion BamHI; Lane 3 - 4: pFastBac1-ORF2-NSP4 single digestion SalI; and Lane 5-6: pFastBac1-ORF2-NSP4 double digestion BamHI/SalI.
4.2. Subcloning the Truncated ORF2 in pFastBac1
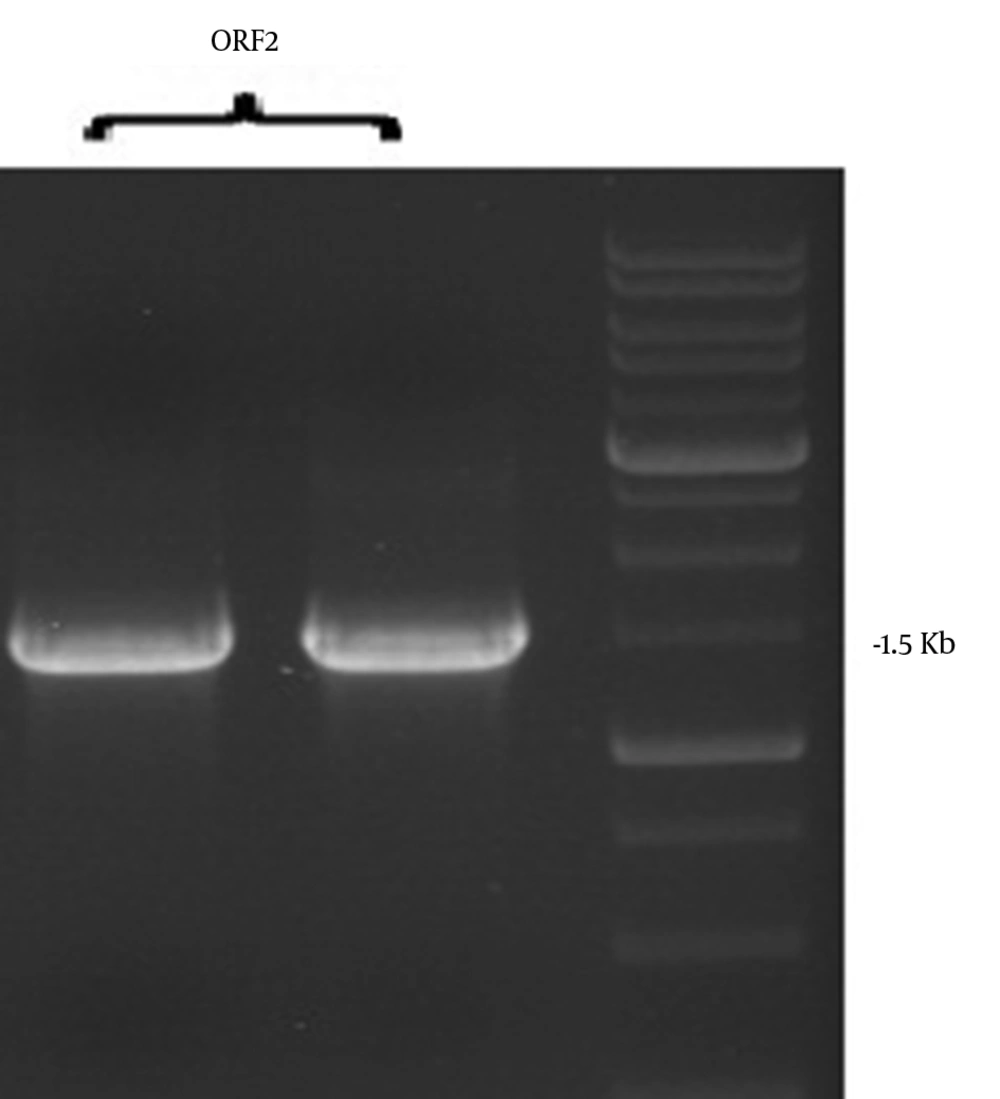
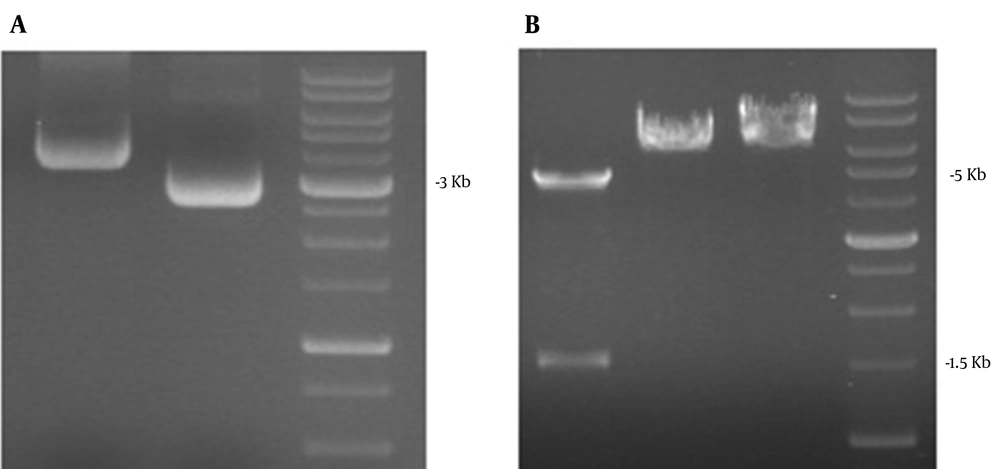
As shown in Figures 2 and 3, the ORF2 truncated gene from pBlueScript ORF2-NSP4 was amplified by PCR (1500 bp) followed by double digestion and sub cloning in linearized PfastBac1.The final size of the subcloned gene was 6275bp as confirmed by single and double digestion using BamHI/SalI.
4.3. Confirmation of Subcloning
The presence of truncated ORF2 and truncated ORF2-NSP4 in the PfastBac1 plasmid was confirmed according to the sequencing report.
4.4. Retransformation of Genes Into DH10Bac
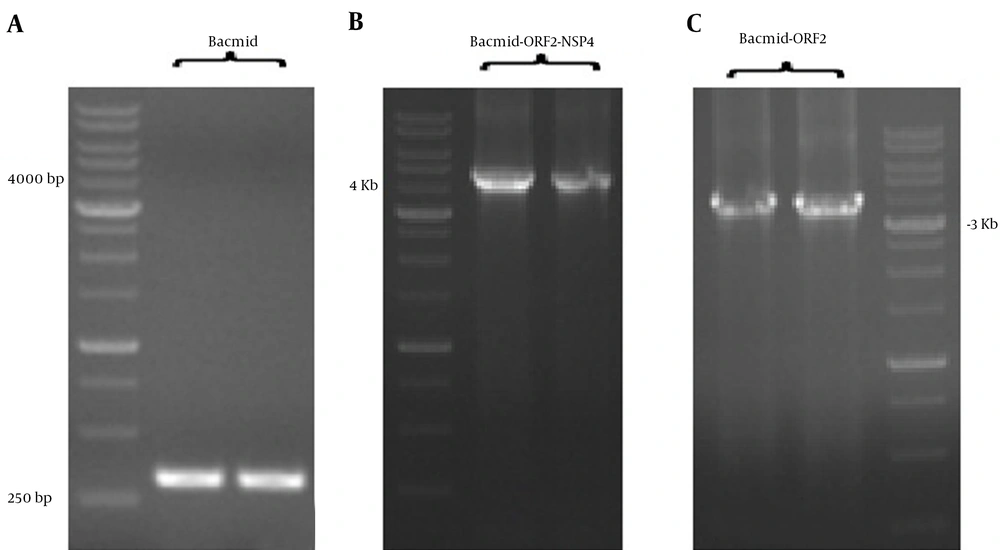
The pfast Back 1 + truncated ORF2-NSP4 and pfast Back 1 + truncated ORF2 genes were transformed in DH10Bac containing the helper plasmid. The length of Bacmid DNA PCR product without recombination was 300 bp and the lengths of Bacmid truncated ORF2-NSP4 and Bacmid truncated ORF2 PCR products were 4300 bp and 3800 bp, respectively (Figure 4).
4.5. Expression of Truncated ORF2-NSP4 and Truncated ORF2 Proteins in SF9 Cells
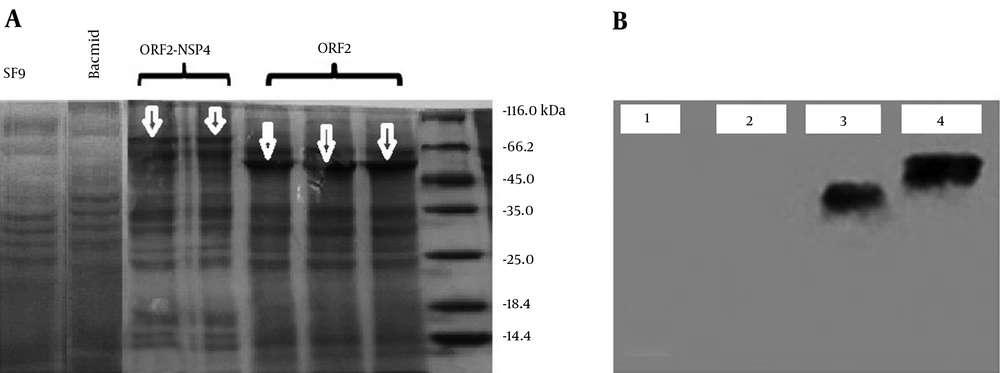
The SDS-PAGE analyses of truncated ORF2-NSP4 and truncated ORF2 proteins showed the expressed proteins were seen at about 56 KDa and 74.5 KDa. To confirm SDS-PAGE results, western blot with specific antibody was carried out. The sizes of expressed proteins (truncated ORF2-NSP4 and truncated ORF2 proteins) in baculovirus expression system were 74.5 and 56KDa, respectively (Figure 5).
A: Analysis of the expression of truncated ORF2-NSP4 and truncated ORF2 proteins by Sodium Dodecyl Sulphate-Polyacrylmide Gel Electrophoresis. The expressed proteins (truncated ORF2-NSP4 and truncated ORF2 proteins) were analyzed by 12% SDS-PAGE and stained with Coomassie Brilliant Blue. The truncated ORF2 and truncated ORF2-NSP4 proteins were respectively seen at about 56 KDa and 74.5 KDa using a Pierce TM Unstained Protein MW Marker (Thermo Fisher Scientific, USA). B: Expression of truncated ORF2-NSP4 and truncated ORF2 proteins in SF9 cells. The analysis of western blot results showed the size of expressed proteins (truncated ORF2 and truncated ORF2-NSP4 proteins) in baculovirus expression system which were respectively 56 KDa and 74.5 KDa. 1: SF9; 2: Bacmid; 3: Truncated ORF-2; and 4: Truncated ORF2-NSP-4.
5. Discussion
The HEV infection is a water borne disease which can cause epidemics in endemic regions, such as Asia, Africa and some parts of South America. Sporadic forms also occur in modern countries or developing countries. HEV has five genotypes: genotype I is dominant in Asia and Africa, genotype II in United States, genotype III in Mexico, genotype IV in Beijing, China and genotype V in Europe (16). In this study, the constructed recombinant truncated ORF2 (112 - 607)-NSP4 (OSU-a) and truncated ORF2 were successfully expressed in the baculovirus expression system.
It has been shown that codon optimization in the baculovirus based expression system could enhance expression of recombinant proteins for primarily designed influenza, HIV and papilloma viruses vaccines (17-19). Consistent with the previous reports (17-19), the present study showed that the codon optimization for recombinant truncated OFR2-NSP4 and truncated ORF2 genes were effectively expressed in SF9 cells.
Many efforts have been conducted to develop an effective vaccine against the hepatitis E infection (1, 5). ORF2 of HEV, a 72-kDa capsid protein, is the major protein of virion. It has been shown that the ORF2-induced antibody in humans and animals is a long-lived, cross-reactive among diverse HEV genotype and could neutralize HEV in vitro. Due to its effective immunogenicity, the HEV ORF2 protein was used as an antigen for all vaccine studies until now. Many expression systems including insect, bacterial, yeast, animal and plant cells have ability to express the full-length and truncated forms of ORF2 protein (5).
Among recombinant protein expression systems, the baculovirus expression system has capability to produce high level recombinant proteins and induced modifications such as folding, oligomerization, phosphorylation, glycosylation, acylation, disulfide bond formation and proteolytic cleavage, without affecting the biological activity of original proteins (20). Therefore, the baculovirus expression system is a highly efficient system for developing vaccines.
The E. coli expressed HEV 239 protein (Hecolin) and baculovirus expressed 56 kDa protein (Novavax) are the only two vaccine candidates that have progressed to stage of clinical trials (5). The under clinical trialed ORF2 vaccine (a 56KDa protein expressed in baculovirus expression system) used alum as an adjuvant (12). It has been demonstrated that alum has local and systemic side effects such as eosinophilia, sterile abscesses and myofascitis (21). Therefore, using a new adjuvant in vaccine construction with less side effects and higher ability for induction of mucosal immunity seems to be important.
Recently, stimulation of the mucosal immunization system through mucosal adjuvants has become important due to the fact that this system is capable to induce immunity in mucosal surfaces against all pathogens that can attach, colonize at mucosal epithelium, or penetrate and replicate in the mucosa (13). Concerning synthesizing a vaccine capable of inducing mucosal immunity, we constructed a new mucosal adjuvant in designing HEV vaccine. To achieve this goal, in the current study a mutant form of OSU-a strain of NSP4 of the Rotavirus linked to C-terminal of the truncated ORF2 protein of HEV as an adjuvant. NSP4 has been shown to exert multifunctional activity and a potential immune response effect. Also, it has been shown that antibody against NSP4 decreases the rate of Rotavirus-induced diarrhea in rabbits (15). Moreover, the adjuvancity of NSP4 in the Rotavirus vaccine is well established (14). It has been shown that repeated passage of virulent strain of NSP4 (OSU-v) in tissue culture developed a strain (OSU-a) with least pathogenicity (22). Therefore, these findings suggest that NSP4 is a suitable protein candidate in vaccine designing.
Finally, the results of the present study suggested that regarding to the oral-fecal route of transmission of both HEV and Rotavirus (3), the ability of NSP4 in inducing protective immune response (15), development a vaccine to induce mucosal immunity and prevent both infections may be useful especially in endemic regions. However, this study only evaluated feasibility of the baculovirus expression system to express the truncated ORF2-NSP4 protein while inducing the immune response by truncated ORF2-NSP4 protein remains to be investigated. In conclusion, the current study showed that the truncated ORF2 and truncated ORF2-NSP4 proteins is successfully expressed in the baculovirus expression system and could be a potential candidate for developing a hepatitis E and Rotavirus vaccine.