1. Background
Occult hepatitis B infection (OBI) is detected by finding HBV DNA in the liver (with detectable or undetectable HBV DNA in the serum) in patients with negative tests for HBV surface antigen (HBsAg). The gold standard for diagnosis of OBI is liver biopsy and detecting the presence of HBV DNA in hepatocytes (1-3). An alternative method for diagnosis of OBI is detecting HBV DNA in peripheral blood samples. Patients who recover from self-limited acute hepatitis B infection without any clinical or biochemical signs of liver damage may develop OBI (4-6). Occult hepatitis B infection can lead to cirrhosis and fibrosis, and it is also an important risk factor for developing hepatocellular carcinoma in HCV-infected and HCV-negative patients with chronic liver disease (7, 8).
Occult hepatitis B infection is more common in patients with HCV or HIV (9, 10) and in areas with a high prevalence of HBV (11). Its prevalence in patients with CHC varies from 30% to 50% (12-15). OBI has been also reported in hemodialysis patients with CHC (16). It has been reported that the risk of primary liver cancer caused by HCV infection may increase if there is coinfection of OBI and CHC (17). Additionally, cirrhosis (15), elevated serum alanine aminotransferase (ALT) levels, and high histological activity (13, 18) are more common in these patients. These results suggest that OBI with CHC may have synergistic effects in the development of liver disease.
One important mechanism for transmission of HBV and HCV is blood transfusions. Due to this transmission risk, all blood units and their components in blood transfusion services are screened for HBsAg. Patients with OBI can transmit HBV via blood transfusion and organ transplantation (19, 20). Occult hepatitis B infection is spread widely throughout the world and epidemiological, geographical, and ethnic factors, as well as risk factors such as CHC (13), may contribute to the prevalence of OBI (21). It is well established that OBI carriers can be the source of HBV in blood recipients. In other words, it seems that the most likely cause of post-transfusion hepatitis in patients who are on hemodialysis or have hemophilia or thalassemia is OBI (10, 22). Patients with thalassemia and hemophilia are at risk because they receive large volumes of blood and blood components (21). For this reason, some studies have evaluated the prevalence of OBI in hemophilia and thalassemia patients. The prevalence of OBI among hemophilia patients has been reported to the range from zero (23) to 51.2% (24) and in patients with thalassemia from zero (21) to 31.4% (25). Therefore, the prevalence of OBI is high in these patients. In addition, it seems that there is a lack of available information to design plans for diagnosis and treatment of OBI in these patients. As mentioned previously, one of the most important risk factors for the spread of OBI is CHC (13). Furthermore, HCV is transmitted by blood transfusion and is frequently seen in hemophilia and thalassemia patients (26).
2. Objectives
We designed this study to assess the prevalence of OBI among Iranian patients with hematological disorders (thalassemia, hemophilia and some other coagulation factor deficiencies) infected with CHC. We also evaluated the need for OBI screening in blood transfusion centers.
3. Methods
3.1. Study Design, Population and Setting
In this descriptive cross-sectional study, all patients with hematological disorders (thalassemia, hemophilia or other coagulation factor deficiencies) referred to the Tehran Hepatitis Center (TCH) between 2009 and 2010 were enrolled. Positive tests for HCV-RNA and anti-HCV and negative tests for HBsAg and human immunodeficiency virus (HIV) antibody were inclusion criteria.
3.2. Ethics Approval
This study was approved by the Baqiyatallah University of Medical Sciences (BMSU) ethics committee (IR.BMSU.REC.1388.20). Each patient signed an informed consent form when enrolled to the study.
3.3. Data Collection
A questionnaire was completed for each patient that included demographic information (age, gender), past medical history (Hepatitis B infection and other diseases), history of transfusion, and family history of hepatitis and history of vaccination against hepatitis B. Patients who were known to have hepatitis B infection were excluded from the study. Blood samples were sent to one laboratory; hepatitis B viral surface (HBS) Ab, hepatitis B viral core (HBC) Ab, HCV viral load, HCV genotyping and HBV DNA were checked for each patient.
3.4. Serology and Molecular Assessment
HBsAg, anti-HBsAb, HBc Ab were tested with ELISA (Radim SpA, Rome, Italy). OBI identification was based on serum HBV-DNA detection. For detection of HBV-DNA, plasma sample was collected from patients and stored in -80°C. HBV-DNA was extracted from sample using High Pure Viral Nucleic Acid Kit (Roche, Penzeberg, Germany) according to manufacturer’s instructions.
The extracted nucleic acid was subjected to (polymerase chain reaction) using HBV FLASH PCR Detection Kit (DNA-Technology, Moscow, Russia) according to manufacturer’s instructions.
3.5. Data Analysis
Data were analyzed using SPSS software v.16 for Windows (SPSS Inc., Chicago, IL, USA). The results were reported as the mean ± standard deviation (SD) for the continuous variables and frequency (percentage) for categorical variables.
4. Results
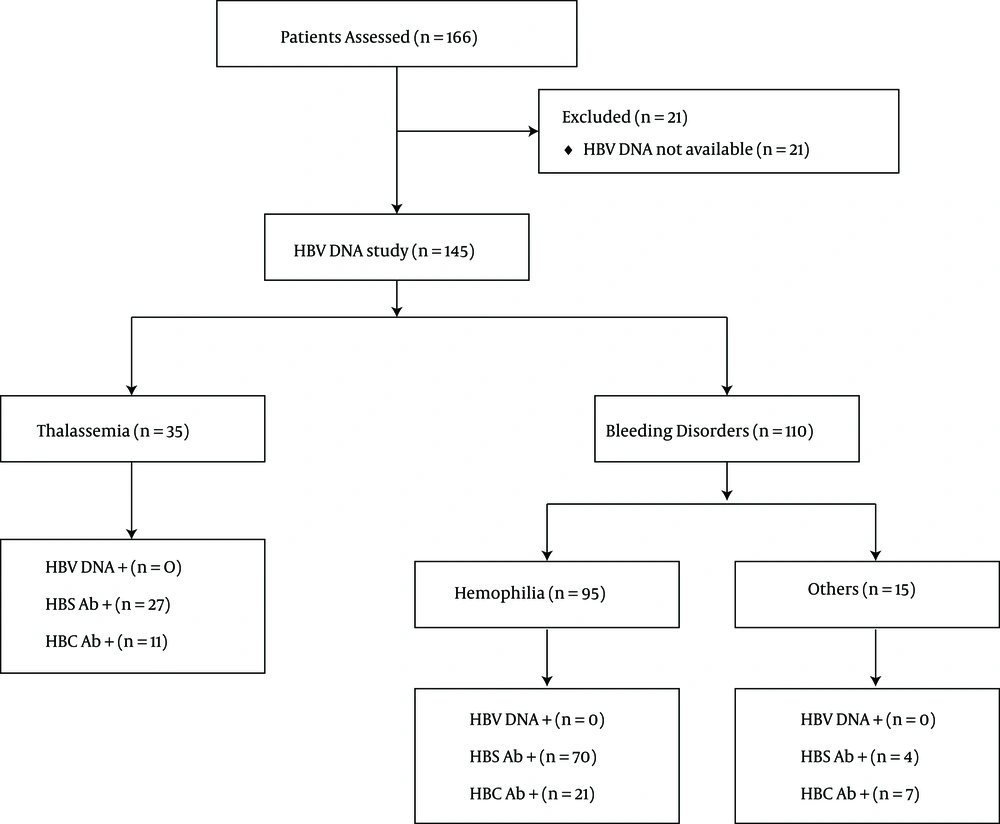
166 chronic HCV patients were evaluated (Figure 1). All of the patients were HBsAg negative and HCV RNA-positive. Only 145 patients were evaluated for HBV DNA (126 men and 19 women). The mean age was 28.12 ± 8.6 years. Thirty-five patients had thalassemia, 95 patients had hemophilia, and 15 patients had coagulation factor deficiencies. The most prevalent HCV genotype was type 1a and the mean viral load was 11.71 × 105 copies/mL. Patients are described in Table 1.
| Description | Valuesa |
|---|---|
| Male Gender | 126 (86.9) |
| Age, y | 28.12 ± 7.6 |
| Thalassemia | 35 (24.2) |
| Hemophilia | 95 (65.5) |
| Factors deficiency | 15 (10.3) |
| HCV genotype | |
| Type 1a | 78 (53.8) |
| Type 1b | 9 (6.2) |
| Type 2a | 1 (0.7) |
| Type 3a | 49 (33.8) |
| Type 4a | 2 (4.1) |
| Blood transfusion | 145 (100) |
aValues are expressed as mean ± SD or No. (%).
The mean age was 24.21 ± 5.1 years in patients with thalassemia and 29.12 ± 8.4 in patients with bleeding disorders. Thalassemia patients were more HBV-vaccinated in comparison with patients with bleeding disorders (88.6% vs. 50%, P < 0.001). The viral load was higher in patients with bleeding disorders (8.3 × 105 vs. 1.28 × 106 copies/mL, P = 0.003). The prevalence of HBS-Ab- and HBC-Ab-positive tests was higher in patients with thalassemia compared with patients with bleeding disorders; this difference was not statistically significant (P > 0.05). Thalassemia patients are described in Table 2 patients with bleeding disorders are described in Table 3.
| Description | Valuesa |
|---|---|
| Male Gender | 26 (74.3) |
| Age, y | 24.21 ± 5.1 |
| Major Thalassemia | 30 (85.7) |
| Intermediate Thalassemia | 5 (14.3) |
| HBV Vaccination | 31 (88.6) |
| Splenectomy | 26 (74.3) |
| Viral Load, *105 copy | 8.3 ± 7.6 |
| HBS Ab Positive | 27 (77.1) |
| HBC Ab Positive | 11 (31.4) |
| HBV DNA Positive | 0 (0) |
aValues are expressed as mean ± SD or No. (%).
| Description | Valuesa |
|---|---|
| Male gender | 105 (95.5) |
| Age, y | 29.12 ± 8.4 |
| Type A hemophilia | 75 (68.2) |
| Type B hemophilia | 20 (18.2) |
| Other bleeding disorders | 15 (13.6) |
| Von Willberand | 5 (4.5) |
| Deficiency factor XIII | 2 (1.8) |
| Glanzman syndrome | 4 (3.6) |
| Deficiency Factor XII | 1 (0.9) |
| Deficiency Factor Xi, XIII | 1 (0.9) |
| Deficiency Factor II | 1 (0.9) |
| Deficiency Factor X | 1 (0.9) |
| HBV Vaccination | 55 (50) |
| Splenectomy | 0 (0) |
| Viral Load, *106 copy | 1.28 ± 11 |
| HBS Ab Positive | 74 (67.3) |
| HBC Ab Positive | 28 (25.8) |
| HBV DNA Positive | 0 (0) |
aValues are expressed as mean ± SD or No. (%).
5. Discussion
To the best of our knowledge, this is the first study that investigated the OBI prevalence rate among patients with hematological disorders (thalassemia, hemophilia and other coagulation factor deficiencies) who were all infected with CHC and were negative for HBs Ag. Because of the particular diagnostic tests for OBI, it is not diagnosed during common screening in blood transfusion centers and can therefore be transmitted via transfusion of infected blood or blood components and induce post transfusion hepatitis (PTH) (27, 28). These conditions should be considered among patients who need permanent transfusions of blood, as in the case of hemophilia, thalassemia and hemodialysis patients (29, 30). In addition, OBI is observed more frequently in patients with CHC infection. Among CHC patients, OBI is considered to be a risk factor for cirrhosis, HCC, and lower survival rates. OBI and HCV can be transmitted via blood (8, 31). Therefore, co-infection of OBI and HCV in thalassemic and hemophilic patients is a very important issue for blood transfusion centers and for their screening methods. Here, based on our results, we did not find any OBI cases in our patients. There are a few studies in the literature that investigate the prevalence of OBI among patients with hematological disorders, such as hemophilia and thalassemia, particularly those infected with HCV (Tables 4 and 5). In our study, we evaluated 15 patients with coagulation factor deficiencies (Table 3). All of these patients also had CHC, but we found no cases of OBI among them. However, patients with coagulation factor deficiencies, especially with CHC, need to be more evaluated in future studies with larger sample sizes.
Abbreviations: HBc, Hepatitis B viral core; HBS, Hepatitis B viral surface; HCV, Hepatitis C virus; ND, not determinant; OBI, occult hepatitis B infection.
Abbreviations: HBc, Hepatitis B viral core; HBS, Hepatitis B viral surface; HCV, Hepatitis C virus; ND, not determinant; OBI, occult hepatitis B infection.
aPrevalence of Anti HBc and Anti HBs in this study is just mentioned in HCV infected patients.
5.1. Occult Hepatitis B Infection and Hemophilia
In our project, we found no cases of OBI in 35 hemophilia patients infected with HCV. In contrast to our results, Toyoda et al. in 2004 evaluated 43 hemophilia patients and reported OBI in 22 of them (51.2%). It should be considered that a high prevalence of transfusion-transmitted infections had been reported in their patients. However, they did not report clinically significant implications for OBI in these patients (24). In 2004, Borhany et al. showed that the rate of OBI prevalence in patients with hemophilia was 1.73% in Pakistan. However, they found no co-infection between OBI and HCV in these patients (32). On the other hand, and similar to our results, 115 Polish hemophilia patients were investigated for OBI in 2006, and no cases of OBI were found. However, HBV DNA was found in nine subjects who were all positive for HBs Ag, and six were HCV-RNA-positive (23). As one of the advantages of our study, it should be noted that HCV infection was an inclusion criterion for our study sample. However, in these mentioned studies, some or all of their cases did not have HCV infection.
5.2. Occult Hepatitis B Infection and Thalassemia
Thalassemia patients are at risk for co-infection with HBV and HCV (35). In our study, we did not find HBV DNA in the sera of 95 evaluated thalassemia patients. In contrast to our study, Shaker and coworkers evaluated 80 Egyptian thalassemia patients and found an OBI prevalence of 32.5% (26 patients). Twenty cases of their whole study group were also positive for both HCV RNA and HBV DNA (33). Similar to our results, Arababad et al. found that none of 60 evaluated thalassemic patients had OBI. However, they reported that only 27 cases were positive for HCV-RNA and that anti-HBc and anti-HBs were positive in 9 and 11 subjects (out of 27), respectively (21). An Indian study in 2003 showed that the rate of OBI prevalence was 31.4% among 70 thalassemic patents (25). On the other hand, Sabat et al. in 2014 reported that the prevalence of OBI in Indian thalassemic patients was 4.5% (34). However, the rate of HCV prevalence between these two studies was different, and they did not determine the OBI prevalence in HCV-infected patients (Table 5).
5.3. Possible Reasons for Different Reported Prevalence of OBI
Differences in reported prevalence of OBI in different countries can be related to the accuracy and performance of OBI diagnostic tests. The serum level of HBV DNA in OBI patients is very low compared with HBs Ag-positive patients. Therefore, it is said that the HBV DNA test should be conducted three times to reduce the false-negative results of the test and to increase the chances of detecting HBV DNA with PCR. When two of these three tests are positive, OBI is definitively diagnosed. However, due to the fact that the HBV DNA level in the sera of OBI cases is very low, many OBI patients cannot be diagnosed with this method and in these cases, liver biopsy is more helpful (36, 37).
Another factor that may contribute to the different prevalence rates of OBI is that developed countries like Japan have more powerful surveillance systems in their blood transfusion centers and health services. This can lead to more cases of OBI being reported, which may be similar to the prevalence of OBI in hemophilia patients (30). Differences in sample size, geographical area (due to the prevalence of HBV) and diagnostic techniques are other related factors in this issue (37).
There are some unique aspects of our study that may have had an effect on the OBI prevalence rate. First, as we have said, despite other studies in which some of the patients had HCV infection, all of our cases had CHC infection. Second, our study sample was HBs Ag-negative. In other words, we selected those thalassemic and hemophilic patients with CHC infection and negative HBs Ag and evaluated them for OBI. These two important points were necessary for our project goals. A question can be raised here: is it possible that CHC can reduce the rate of identification of OBI in thalassemic and hemophilic patients? We think that using more accurate diagnostic tests, such as liver biopsies, in future studies could be helpful for answering this question.
5.4. Conclusions
In this study, we found no OBI cases in the chronic HCV-infected patients with thalassemia and bleeding disorders, especially hemophilia. However, to make better decisions about OBI screening, particularly in transfusion centers, and about the use of a comprehensive screening method, more original studies with more precise laboratory techniques and larger sample sizes are still needed.