1. Background
Hepatitis E virus (HEV) is one of the causative agents of acute or fulminant hepatitis, which is prevalent in most developing countries, principally as a water-borne infection in resource-limited areas with limited access to essential water. The total mortality rate of HEV during outbreaks range from 1% to 15% in the general population and the highest mortality occurs in pregnant women, with fatality rates of up to 30% (1, 2). Most commonly, HEV is transmitted by the fecal-oral route, however, the unusual way has been also reported (3). Although HEV is classified into 8 genotypes, only one serotype has been characterized so far (4), indicating promising consideration for vaccine construction. Since, there is no suitable cell culture system for efficient HEV replication and susceptible small laboratory animal, construction of live/attenuated vaccine is not possible. Hepatitis E virus has 3 overlapping open reading frames (ORF1-3), ORF2 encodes capsid protein, which has the main neutralization epitopes (NE) (5). One region in c-terminal of ORF2 gene, so called HEV 239 (ORF2 368 - 606 aa) has been shown to be related to the viral antigenicity and immunogenicity (6). Recent efforts have mostly focused on the development of recombinant vaccines based on the ORF-2 capsid protein (7).
The first recombinant HEV vaccine, HEV239 (Hecolin; Xiamen Innovax Biotech, Xiamen, China), was approved by the State Food and Drug Administration (SFDA) in China for use in the general population (8). HEV239, as a prophylactic vaccine, can induce excellent protective immunity in healthy adults; however, the efficacy of this recombinant protein-based vaccine has not yet been tested for high-risk groups, such as pregnant women, children, and immunocompromised individuals (9). DNA vaccines are an effective method to generate antigen-specific humoral and cell-mediated immune responses; they are relatively safe, cost-effective, easily produced, and are able to sustain reasonable intracellular levels of antigen expression (10). However, limited immunogenicity has evidenced a significant obstacle for efficacy of DNA vaccines, especially in higher primates and humans. To date, several approaches have been taken to enhance the potency of such vaccines (11).
Autophagy is a continuous biological process that can enfold cytoplasmic material in a cup-shaped double membrane construction, known as autophagosome and subsequently recycled (12). Its essential role in both innate and adaptive immunity has been confirmed. Recently, it has been determined that autophagy plays a significant role in cellular immunity against intracellular pathogens such as viruses or some bacteria by enclosing and targeting them for removal function through the delivery of the antigens to major histocompatibility complex class I and II (MHC-I and-II) presentation (13, 14).
Employing of pharmacological or immunological methods for activation of autophagy can be used for promoting protective efficacy of vaccines (15). Beclin-1 has a critical role in coordinating the cytoprotective function of autophagy and it is the first recognized mammalian autophagy-related gene to trigger autophagy. It interacts with several cofactors to induce degradation and turnover of macromolecules and organelles in autophagy process (16, 17). In some cases such as stress or nutrient depletion, when autophagy is triggered, Beclin-1 forms a multiprotein complex with class III phosphatidylinositol 3-kinase (PI3K) responsible for the initiation of the isolation membrane (18). Beclin-1 also marks the membrane for autophagosome formation and promotes autophagosome fusion with lysosomes (19).
2. Objectives
Based on the important role of autophagy in pathogens antigen presentation, it is tempting to speculate that it may be possible to activate autophagy as a novel DNA vaccine strategy against human infectious diseases. We hypothesized that incorporating an autophagy-inducing plasmid into a DNA vaccine improves efficacy of DNA vaccine. In the present study, the function of Beclin-1 incorporating into a HEV239 DNA vaccine as a novel model was evaluated in the mice model.
3. Methods
3.1. Plasmid Construction and Production of Recombinant HEV239
In order to construct the plasmid, the 368 - 606 amino acid sequences of ORF-2 of HEV (SAR-55 strain) and the 1 - 450 amino acid sequences of Beclin-1 were retrieved from Genbank databases with the GenBank accession numbers (“AAA45727.1 and NP-003757”). The synthetic genes were flanked by BamHI and SalI for Beclin-1 to facilitate subcloning. Moreover, recognition sequences for restriction enzymes (SalI and XhoI) were added at the beginning of the HEV239 gene; also, NheI and SmaI restriction sites were placed at the end of gene for subcloning into PUC19 and pVITRO, respectively. The genes were synthesized by Bio Basic Inc. Ontario, Canada and cloned into the Puc19 cloning vector. Furthermore, in order to express efficiently in prokaryotic cell, the codon optimization was performed for HEV239 gene and for increasing the efficiency of translation initiation, the Kozak sequences were placed in upstream of the both genes. For purification of protein and confirmation of gene expression, a polyhistidine-tag was added on the 5’ end of the genes.
For construction of the plasmid harboring HEV239 and Beclin1, the synthetized Beclin-1 was digested with the BamHI and SalI from Puc19. Then, they sub-cloned into multiple cloning site 1 (MCS1), which XhoI and NheI restriction enzymes were selected for sub-cloning synthetized HEV239 into MCS2 of the eukaryotic expression vector pVITRO2-neo-mcs (InvivoGen). All subcloning products were confirmed by sequencing. Bacterial strain Escherichia coli DH5 α (Pasteur Institute of Iran) was used for propagation and preparation of the pVITRO2 (InvivoGen) plasmid. For production of recombinant HEV239, the HEV239 was subcloned into the plasmid Pet26b (Novagen). The HEV239 protein was purified using Ni-NTA purification system (Qiagen, Hilden, Germany), according to the manufacturer’s instructions (data are not shown).
3.2. Cell Transfection
HEK293 cell line with Dulbecco modified Eagle medium (DMEM) supplemented with L-glutamine, 10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin were incubated at 37.0°C in 5% CO2. To measure autophagy, pVITRO, pVITRO-HEV239, pVITRO-Beclin1, and pVITRO-HEV239-Beclin1 plasmids were transfected into HEK293 cells by Lipofectamine 3000 transfection reagent according to the manufacturer’s instructions (Invitrogen). Briefly, the 5 µg of DNA and 10 µL of P3000TM solution were diluted in 250 µL of Opti-MEM without serum. After 5 minutes of incubation, the diluted DNA was combined with the diluted Lipofectamine 3000 and incubated for 5 minutes at room temperature (RT). Then, the mixture was added to each well and mixed gently. The cells were incubated at 37°C in a CO2 incubator for 48 hours until they are ready to assay for transgene expression. All experiments were performed in triplicate.
3.3. RNA Isolation and Quantitative Real-Time PCR
Total RNA was isolated by RNeasy plus Mini Kit (Qiagen, Germany), according to the manufacturer’s instructions, 48 hours post-transfection. DNase treatment was performed using the on-column DNase digestion (Qiagen, Germany). A total of 10 µg of total RNA was reverse transcribed using M-MulV Reverse Transcriptase with specific primer for the first-strand cDNA synthesis. It was subjected to real time quantitative PCR in triplicate on the Rotor-Gene 6000 (Corbett Life Science, Australia) and EvaGreen in qPCR master mix kit (YTA, Iran). Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) gene was considered as the reference gene for normalization of relative quantification. All designed primers were confirmed using the NCBI Blast tool against all available mRNA sequences to ensure specificity (Table 1).
| Number | Gene | Primer Sequence(5’ - 3’) | Length of Primer |
|---|---|---|---|
| 1 | Beclin-1 | GGAGAGACCCAGGAGGAAGAG | 21 |
| 2 | Beclin-1 | GCCTCCCCAATCAGAGTGAAG | 21 |
| 3 | GAPDH | CTCTGCTCCTCCTGTTCGAC | 20 |
| 4 | GAPDH | TTAAAAGCAGCCCTGGTGAC | 20 |
3.4. Western Blotting
For western blotting, proteins in cell lysates were boiled for 5 minutes followed by resolving and separating onto a 10 - 15% SDS-PAGE. Furthermore, HEV239 proteins were transferred onto nitrocellulose membranes (Amersham, United Kingdom). Later, the membrane was blocked with a blocking buffer containing 5% skimmed milk plus 0.05% between 20 for 3 hours in RT. It was followed by immunoblotting with the primary monoclonal antibodies (mAb): anti-6X His tag mAb (dilution, 1:1000; Abcam, Cambridge, UK), or anti-beta actin antibody (Abcam, Cambridge, UK) over night at 4°C. After washing, the membranes were incubated with goat anti mouse immunoglobulin G-horseradish peroxidase secondary antibody (dilution, 1:5000; Santa Cruz Biotechnology) for 1 hour at RT. Ultimately, proteins were visualized using an enhanced chemiluminescence (ECL) purchased from Amersham company.
3.5. Immunization Protocol and Vaccine Preparation
Eight-week-old female BALB/c mice were purchased from the institute pasteur of Iran (Karaj, Iran). All animal experiments were performed in accordance with the confirmed animal protocols of the Institute Pasteur of Iran. Mice were assigned randomly into 5 groups (8 mice were used for each plasmid DNA vaccination): pVITRO-HEV239 alone, pVITRO-HEV239-Beclin-1, pVITRO-Beclin-1, only pVITRO empty vector, and PBS. The mice were injected by subcutaneous injection with 100 µg of naked plasmid DNA 3 times at days 0, 14, 21.
3.6. Anti-HEV239 Antibodies Detection
Two weeks after the final immunization, mice sera were collected and tested for anti-HEV239 antibodies detection by the ELISA method with purified recombinant HEV239 protein. Flat-bottom 96-well plates were incubated with 10 µg/mL of recombinant HEV239 protein overnight at 4°C. Then, coating solution was removed and individual serum samples were added to each of the coated wells in duplicates and then incubated for 2 hours at RT. After 3 washes with the washing buffer, goat anti-mouse IgG1, IgG2a, and total IgG (1:1,000 in PBS, Sigma, St. Louis, MO, USA) were added to each well and incubated for 30 minutes at room temperature. Next, rabbit anti-Goat IgG HRP conjugate (1:5,000 in washing buffer) was added after 3 washes and incubation was performed for 15 minutes. In order to determine a color reaction, peroxidase substrate was added and at the end of incubation time, the reaction was stopped with 3N NaOH. Finally, the optical density (OD) was calculated at 450 nm (Stat Fax 4200 Microplate Reader).
3.7. Cytokine Release and Lymphocyte Proliferation Assay
In order to assay lymphocyte proliferation, the mice were scarified 2 weeks after the final immunization and the spleens were removed. Cell proliferation ELISA, BrdU (5-bromo-2 deoxyuridine, Roche, Germany) kit was used to assay cell proliferation. Briefly spleen cells (1 × 106 cells/well) were cultured in RPMI1640 and stimulated on the presence of Ag HEV-239 (5 µg/mL) in 96-well flat-bottom plates and incubated for 72 hours at 37°C in 5% CO2. Concanavalin A (Con A) and un-stimulated cells were used for positive and negative controls, respectively. Then, 20 µL BrdU labeling solution was added per well and incubated for 12 hours at 37°C in a CO2 incubator. Finally, Brdu can be detected by anti-BrdU antibodies (Roche, Germany) and an analysis was performed according to the manufacturer’s instructions.
3.8. IFN-γ and IL-4 Enzyme-linked Immunospot (ELISPOT) Assay
The mouse IFN-γ ELISPOT and IL-4 ELISPOT assays were performed using commercial kits provided by MABTECH (Nacka Strand, Sweden), according to the manufacturer’s protocol. Briefly, 96-well ELISPOT plates were coated with 15 µg/mL anti-mouse IFN-γ or anti-mouse IL-4 and incubated overnight at 4 - 8°C. Next, the plates were washed 5 times with 200 µL sterile PBS/well and blocked with 200 µL/well of medium containing 10% of the same serum as used for the cell suspension. Splenocytes from vaccinated and control mice were added in duplicate (2 × 105 cells/well) with recombinant HEV239 protein and incubated at 37°C in the presence of 5% CO2 for 24 hours. Then, the wells were completely washed and incubated with biotinylated anti-mouse IFN-γ or anti-mouse IL-4 for 2 hours at RT. After washing, the wells were incubated with streptavidin-ALP for 1 hour at RT. Wells were washed again and substrate was added to the wells. Color development was monitored until distinct spots emerged and stopped by washing extensively with tap water. After drying overnight at RT, spots of activity were inspected by a dissection stereoscope (Leica microscopy system, Heerbrugg, Switzerland). Data were presented as an average number of spots per 2 × 105 cells ± SD.
3.9. Statistical Analysis
All results were introduced as mean ± standard deviation (SD). Comparisons between groups were conducted by one way ANOVA using GraphPad Prism version 6.00 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com”. Differences were considered significant at a P value less than 0.05.
4. Results
4.1. HEV239 Expression in HEK293 Cells and Induction of Autophagy by Beclin-1
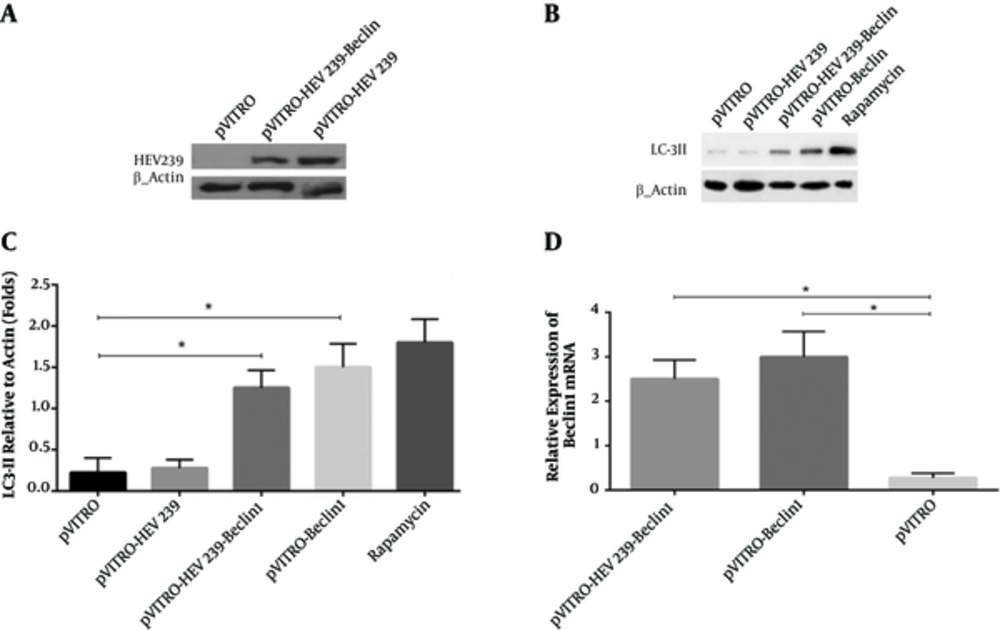
We confirmed HEV239 expression by transfection of the recombinant plasmids pVITRO-HEV239 and pVITRO-HEV239-Beclin-1 into HEK293 cells by western blotting using anti-6X His tag mAb. We observed that HEV239 protein was expressed effectively with clear bands. No HEV239 expression was detected in the cells transfected by pVITRO alone (Figure 1A). To investigate whether Beclin-1 can induce autophagy, we first detected a specific marker for autophagy activation (called LC3-II) in HEK293 cells treated with Beclin-1 or rapamycin by western blot analysis. As shown in Figure 1B, treatment with Beclin1 or rapamycin results in expression of LC3-II as compared to internal control β-actin. In addition, there was a significant higher level of LC3-II in pVITRO-Beclin1 as compared to pVITRO alone (Figure 1C). As a positive control, Rapamycin treatment revealed strong LC3-II accumulation.
A, HEK293 cells were transfected with HEV239-containing plasmids for 48 hours and cell lysates were detected for HEV239 expression by Western blotting. B, Activation of autophagy was analyzed by the presence of LC3 II in transfected cells. Rapamycin-treated cells were used as a positive control. C, LC3-II levels were quantified relative to β-actin. D, Total RNA was collected from transfected HEK293 cells with pVITRO -Beclin1 or pVITRO-HEV239-Beclin1 and quantitative reverse transcription-PCR was used for examination of Beclin1 mRNA expression. Data are demonstrative as the mean ± standard error of 3 independent experiments (*, ** and *** indicate P < 0.05, P < 0.01 and P < 0.001, respectively).
4.2. Up-Regulation of Beclin1 at the Transcriptional Level by Constructed Plasmids
Changes on the expression of Beclin-1 mRNA were confirmed by qRT-PCR in transfected cells. GAPDH mRNA expression was not significantly different between the control and transfected cell lines that also served as an optimal housekeeping gene and results were evaluated by relative quantification and melting curve analysis. In order to investigate whether constructed plasmids enhance Beclin1 gene expression, its regulation at the mRNA level were examined. We observed that transfection with 5 µg/µL of - Beclin-1 and pVITRO-HEV239- Beclin-1 for 48 hours resulted in a 3.1 ± 0.4 (mean ± SD, P < 0.05) and 2.5 ± 0.3 (mean ± SD, P < 0.05) - fold increase of Beclin1 respectively compared to pVITRO-transfected control (Figure 1D).
4.3. Lymphocyte Proliferation Response to HEV239
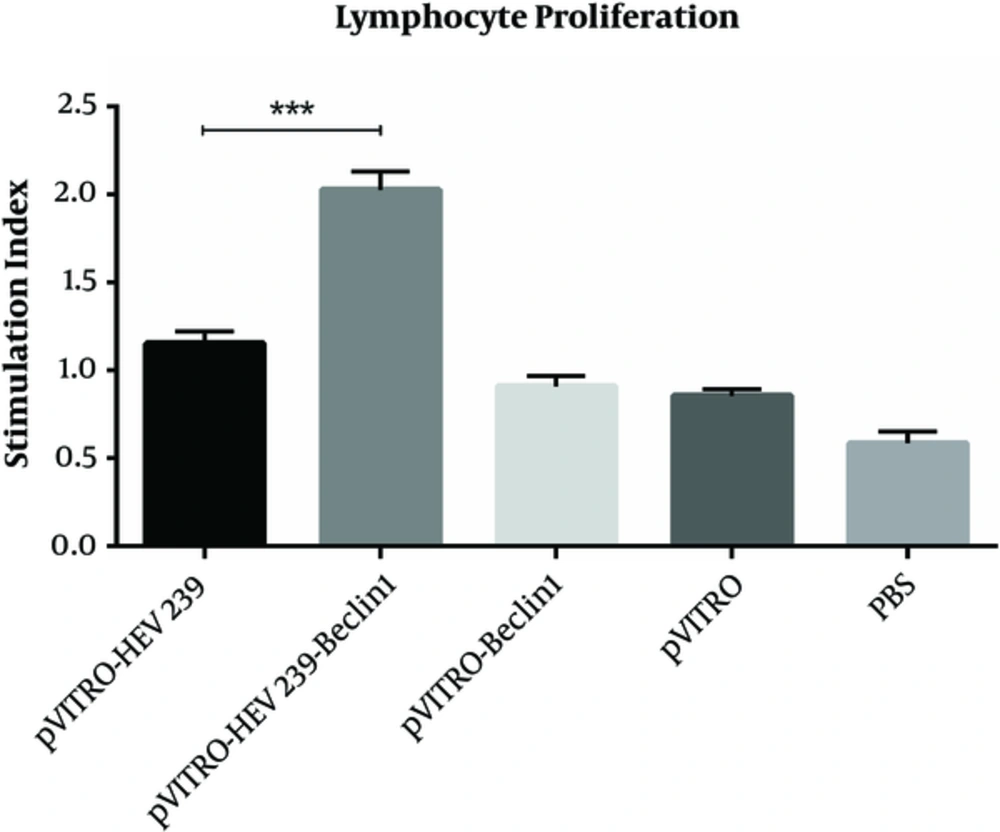
The splenocytes from the immunized mice were cultured and restimulated with HEV239 antigen in vitro 2 weeks after last immunization for the lymphocyte proliferation assay. Significant increases were seen in mice immunized with pVITRO-HEV239- Beclin-1 (SI = 2.02 ± 0.8) in comparison with pVITRO-HEV239 (SI = 1.15 ± 0.5) and control group (pVITRO-Beclin1, Pvitro and PBS) (P < 0.001), suggesting that immunization with pVITRO-HEV239-Beclin-1 based approaches induced cell mediated immune response in BALB/c mice (Figure 2). No significant changes were found between the control groups.
Two weeks after final immunization, spleens of individual mice were removed and lymphocyte proliferation was evaluated by cell proliferation ELISA, BrdU kit. Values are the mean ± standard error for the experiments. The results indicate statistically significant difference between the pVITRO-HEV239- Beclin-1 group as determined by one-way ANOVA (P < 0.001) with other groups (*, ** and *** indicate P < 0.05, P < 0.01 and P < 0.001 respectively).
4.4. Immune Response and Cytokine Production to DNA Vaccine
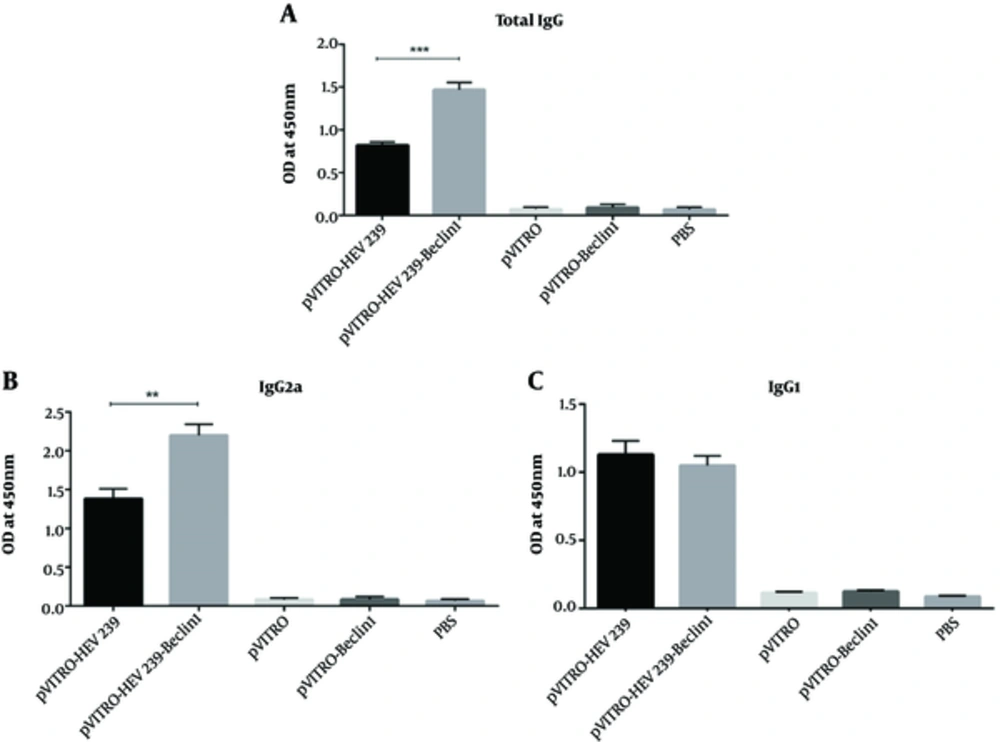
To evaluate the humoral immune response, 2 weeks after the last immunization, anti HEV239 total IgG, IgG2a, and IgG1 levels were measured by ELISA. The group immunized with pVITRO-HEV239-Beclin-1 (autophagy inducing plasmid) indicated higher level of total IgG than in those immunized with pVITRO-HEV239, suggesting that co-expression of Beclin1 with HEV239 in immunization can induce impressive humoral immune response (Figure 3A). Th2 and Th1-type immune responses were examined by IgG1 and IgG2a in mice, respectively. When the titer of specific IgG isotype was evaluated, the group that received autophagy inducing plasmid exhibited the highest IgG2a titer, indicating that Th1 immune response was predominant immunity. No significant changes in IgG1 were detected in the groups immunized with pVITRO-HEV239- Beclin-1 and pVITRO-HEV239. The specific anti-HEV239 antibody titer from mice that vaccinated with pVITRO-Beclin1or empty vector was undetectable (Figure 3B and C).
Serums from pre-immunized mice were used as a negative control. A and B, The IgG2a titers of mice immunized with pVITRO-HEV239-Beclin1 were significantly higher (P < 0.001) than those immunized with pVITRO-HEV239. C, there were no difference in levels of IgG1 between the pVITRO-HEV239-Beclin1 -immunized compared with the pVITRO-HEV239-immunized mice. Statistical significance was analyzed using one-way ANOVA (*, ** and *** indicate P < 0.05, P < 0.01 and P < 0.001 respectively).
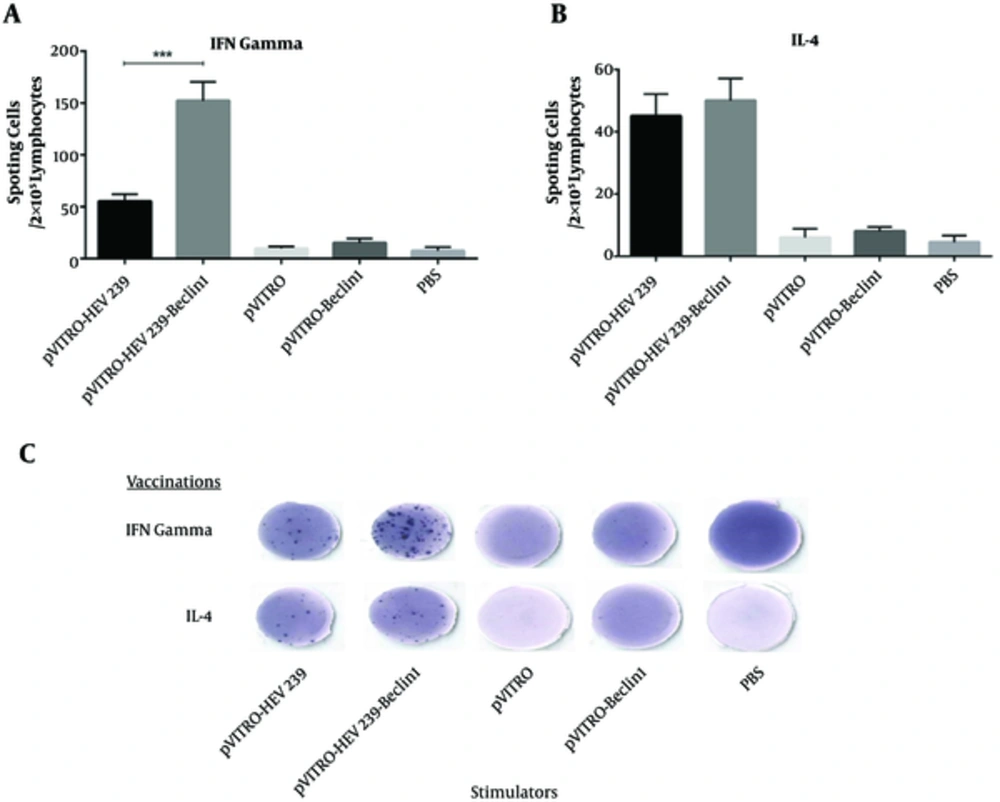
To assay the ability of vectors to produce cytokine, the proportion of IFN-γ and IL-4 between immunized mice and control groups were compared. As shown in Figure 4A, ELISPOT assay revealed that spleen from mice vaccinated with pVITRO-HEV239- Beclin1 produced higher level of IFN-γ in comparison with other groups (P < 0.05), including pVITRO-HEV239 and pVITRO-Beclin1. On the other hand, no significant differences were noted in IL-4 production between pVITRO-HEV239- Beclin1 and pVITRO-HEV239 groups (Figure 4B and C). Taken together, all results definitely confirmed that incorporation of Beclin1 (an autophagy-inducing plasmid) in DNA vaccine formulation induces strong Th1 immune response.
A, Splenocytes from vaccinated and control mice were cultured at the presence of recombinant HEV239 protein and incubated at 37°C for 24 hours. B, Lymphocytes from mice received Beclin1-containing plasmid generated a high number of IFN-γ secreting cells in 2 × 105 lymphocytes. C, no different in IL-4 secreting cells in 2 × 105 lymphocytes were detected in HEV239- Beclin-1 containing plasmid in comparison with pVITRO-HEV239 (*, ** and *** indicate P < 0.05, P < 0.01 and P < 0.001 respectively).
5. Discussion
The progress in traditional inactivated or live-attenuated HEV vaccine development is considerably limited by the lack of an appropriate cell line (20). The major goal of the present study was to examine the function of Beclin-1 gene in increasing the immune response after incorporating into the HEV239 DNA vaccine. As far as we know, this is the first study that makes use of Beclin1 to improve vaccine responses. Several studies have determined the role of autophagy in adaptive antiviral immunity by providing antigens for MHC-II presentation (21, 22). For instance, Schmid et al. demonstrated that targeting influenza matrix protein1 (MP1) to autophagosomes via fusion to the LC3 was able to increase MP1 epitope presentation by MHC class II to CD4+ T cells (14). Autophagy facilitates the presentation of viral-derived antigens to immune system, most viruses develop strategies to inhibit autophagy pathway through binding to Beclin1 as a vital element for initiation of autophagy and the role of Beclin1 has been determined in autophagosome formation and fusion with lysosomes (19). Induction of autophagy pathway by Beclin1 overexpression may be a new strategy to promote induction of antigen-specific CD4+ and CD8+ T cells. As a result, autophagy-inducing based plasmids encoding Beclin1 were constructed to facilitate the autophagic induction in the HEK293 cells. The constructed plasmid induced a significant rise in LC3-II in HEK293 cells after transfection (Figure 1B).
Recent findings suggested that the ORF2 gene could be developed as a potential vaccine candidate against HEV infections (23). Different regions of the ORF2 gene, in several expression systems, have been used for vaccine development (24, 25). The accumulated results of the various studies have identified both B and T cell immunodominant epitopes in HEV 239 and the crucial role of cellular immune responses against hepatitis E have been determined in previous studies (26). Moreover, Tuteja et al. demonstrated that DNA immunization, with the plasmid containing a full-length HEV ORF2, developed low level of long-lasting antibodies to HEV in mice model (27). The HEV239 gene was chosen for construction of HEV candidate vaccine in the present study. To examine the effect of autophagy induction on immune responses in vivo, the mice were injected subcutaneously with HEV239-containing plasmid and a plasmid encoding Beclin1 as an immunostimulatory agent. Strong systemic immune responses were detected after the last immunization in the group receiving pVITRO-HEV239-Beclin1 plasmid.
Following DNA immunization, Beclin1-containing plasmid can enhance T-cell-mediated immunity by cross-presentation. Probably, Beclin1 overexpression forms the antigen-containing autophagosome that subsequently fuses with endocytic compartment and may be presented to CD8+ cells via MHC class I molecules (28). Furthermore, English et al. showed that autophagy facilitated the processing and presentation of HSV-1 antigens on MHC I molecules in infected macrophages (29). We found that co-administration of Beclin1-HEV239 plasmids generated a higher number of antigen- specific IFN-γ+ T cells and elevated a serum level of IgG2a, which was consistent with a greater propensity for Th1 responses. Most possible explanation for this increase in immune response is role of Beclin1 in delivery of HEV239 Ag into MHC class I and II compartment. Hepatitis E is an acute hepatitis and a high titer of neutralizing antibodies had been proved to protect from HEV infection, although the protective antibody level in human beings remains unknown. Our findings demonstrated that incorporation of Beclin1 into DNA vaccination strategies result in increased total IgG and IgG2a in serum, especially in the group co-administrating with pVITRO-HEV239-Beclin1 genes, which is consistent with Th1 type immune responses.
In addition to the Beclin1, other autophagy inducers such as mammalian target of rapamycin- kinase defective (mTOR-KD) and LC3II have also been evaluated as an autophagy inducing system in vaccine development (30, 31). Until now, It had not been tested that overexpression of Beclin1 induced autophagy in DNA vaccine; nevertheless, our study revealed that autophagy is induced through overexpression of this protein. Andersen et al. demonstrated the potential of an autophagy receptor and hypothesized that fusion of the C-terminus of p62 to the human immunodeficiency virus-1 Gagp24 gene would facilitate antigen delivery into the autophagy pathway and greatly enhanced the number of Gag p24-specific IFN-γ-producing T cells and CD8+ T cells in vaccinated mice (22). In parallel to the results of the present study, Meerak et al. revealed that autophagy induction through mTOR-KD could improve the efficacy of a candidate DNA vaccine against mycobacterium tuberculosis (MTB). They found elevated levels of secreted IFN-γ, interleukin-2 (IL-2), and IgG2a in immunized mice with the autophagy-inducing based plasmid (30).
It has earlier been shown that immunization of mice with plasmid DNA expressing full-length or truncated HEV ORF2 elicited antibody and CTL immune responses (32, 33). Our results represented that induction of autophagy promote cell-mediated immune responses in vaccinated mice with HEV239-Beclin1, indicating that incorporating an autophagy-inducing element into a DNA vaccine formulation may be useful to increase a Th1 immune response primarily. Navaneethan et al. indicated that during a pregnancy, due to hormonal changes, absolute shift toward Th2 cells were detected, which may cause a more severe illness and higher mortality rate for pregnant women with HEV infection (34). The outcome of long-term Beclin1 overexpression in cells still remains unknown, however, enhanced autophagy can ultimately induce cell death and the role of these dying cells in processing and presentation of Ag by MHC class I molecules to CD8+ T cells have been determined (22). Although a recombinant HEV239 vaccine had been proved to be efficacious and safe in a phase III clinical trial (8), several recent studies have reported novel and promising strategies for HEV vaccine development (35, 36). However, the value of the current study was to prove the function of Beclin-1 gene after incorporating into a DNA vaccine by using HEV239 DNA vaccine as a model.
6. Conclusions
In conclusion, our observations provide further rationale to the use of Beclin1 as an immunostimulatory agent, not only to increase immunity, but also to stimulate antigen presentation and stronger adaptive immunity response by autophagy induction. Although much more studies are needed to be conducted in order to optimize autophagy pathways, a Beclin-1- mediated autophagy could be used as a novel strategy to improve DNA vaccine potency for other viruses and intracellular pathogens.