1. Background
Acinetobacter baumannii is a nosocomial pathogen, which involves important infections such as pneumonia, endocarditis, surgical infection, urinary tract infection, meningitis, and septicemia. Risk factors for multidrug-resistant A. baumannii infection include prolonged length of hospitalization, exposure to an intensive care unit (ICU), colonization pressure from broad-spectrum antimicrobial therapy, invasive procedures, and underlying disease severity (1, 2). Nosocomial transmission is common in the intensive care units (ICUs), where multidrug-resistant (MDR) A. baumannii can cause invasive disease in critically ill patients. The device-associated A. baumannii infections, especially ventilator-associated pneumonia (VAP), is common in ICUs, results in prolonged hospitalization, and rising healthcare costs. Medical equipment and ICU staff are the primary sources of infection (3).
The centers for disease control and prevention (CDC) 2013 national report indicated that Acinetobacter healthcare-associated infections were 12000 annual cases. Multidrug-resistant isolates were nearly 7000 (or 63%) isolates and about 500 deaths occurred each year. It was responsible for about 2% of nosocomial infections that year, however, the rate of infection among critically ill patients on mechanical ventilators was higher (about 7%) (4). The high rate of resistance to carbapenems, especially imipenem, was reported for nosocomial A. baumannii isolates with the rate of > 50% in Iran, China (58.9%), India (85.7%), Thailand (81.4%), and Malaysia (86.7%) (5-8).
For epidemiological studies, it is useful to monitor the interinstitutional and regional spread of carbapenem-resistant isolates. For that, several molecular typing methods have been developed for typing of A. baumannii isolates. Among those, pulsed-field gel electrophoresis (PFGE) extensively used to determine the relatedness of an organism or to identify the sources of infection (9).
2. Objectives
Available information about the epidemiology of the endemic clones of A. baumannii isolates in Tehran hospitals is very low. Thus, the aim of the present work was to determine the genetic relatedness and antimicrobial susceptibility patterns in the endemic clones of A. baumannii isolates from patients in the ICUs.
3. Methods
3.1. Ethics statement
This study was approved by ethical committee of faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran. (ID. 91356, 2010).
3.2. Bacterial Strains
A total of 55 non-repetitive A. baumannii isolates were selected from among the well-characterized strains collected from various clinical specimens of hospitalized patients in a study conducted during 2010 to 2011 in 2 hospitals of Tehran city (5).
3.3. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing was performed by the disk diffusion method as recommended by the Clinical and Laboratory Standards Institute (CLSI) guidelines. The test antibiotics were as follow: trimethoprim- sulfamethoxazole (23.75 + 1.25 µg), cefepime (30 µg), cefotaxime (30 µg), amikacin (30 µg), imipenem (10 µg), meropenem (10 µg), gentamicin (10 µg), ciprofloxacin (5 µg), aztreonam (30 µg), and ceftazidime (30 µg) (Mast Diagnostics, UK). Escherichia coli ATCC 5922 was used as the quality control strain. Strains resistant to at least 3 classes of antibiotics were considered as MDR strains (10).
3.4. Molecular Detection of Carbapenemase Genes
blaOXA-51-like, blaOXA-23-like, blaOXA-24-like, and blaOXA-58-like genes were detected by a multiplex PCR assay (11). The presence of ISAba1 relative to blaOXA-51 and blaOXA-23 was detected using the primer pairs ISAba1F (5’-CACGAATGCAGAAGTTG-3’) /OXA-51R (5’-CTATAAAATACCTAATTGTT-3’) (expected size 1222 bp), and ISAba1F/OXA-23R (5’-TTAAATAATATTC AGCTGT-3’) (expected size 1456 bp), respectively (12). Amplification of class 1 integrons was performed using published specific primer pairs for intI gene as follows: 5’-CAGTGGACATAAGCCTGTTC-3’ and 5’-CCCGAGGCATAGACTGTA-3’ (amplicon size 160 bp) (13). PCR was performed with a T100TM Thermal Cycler from Bio-Rad and all primers were purchased from Pishgam Co., Iran.
3.5. Genotyping by Pulsed-Field Gel Electrophoresis
Pulsed-field gel electrophoresis was performed using the protocol described by Durmaz et al. (9). Agarose-embedded DNA was digested with ApaI (New England Biolabs) restriction enzyme for 6 hours at 37°C. The digested plugs were run on a 1% low melting agarose gel (Sigma, USA) using a CHEF- Mapper apparatus (Bio-Rad, USA) with initial pulse time of 2.2 seconds and final pulse time of 54.2 seconds for 19 hours at 6 V. The Lambda Ladder PFGE Marker (NEB, US) was used as a molecular size marker. The gels were stained with ethidium bromide and patterns were photographed with UV gel Document (BIO-RAD, USA).
3.6. Statistical analysis
Pulsed-field gel electrophoresis patterns were analyzed by the GelCompar II software program, version 4.0 (Applied Maths, Belgium). The band-based Dice similarity coefficient and the unweight pair group method with arithmetic mean (UPGMA) with settings of 1.5% optimization and 1.5% band position tolerance was used for a dendrogram generation.
4. Results
4.1. Antimicrobial Susceptibility Testing
Antibiotic susceptibility testing showed that 21.8% (12 isolates) were resistant to all tested antibiotics and 1 isolate (156) was susceptible to all tested antibiotics. The highest number of resistances was against aztreonam with 98.1% (54 isolates), 90.9% (50 isolates) were resistant to ceftazidime and cefotaxime, 89% (49 isolates) to ciprofloxacin, 87.2% (48 isolates) to trimethoprim/ sulfamethoxazole, 78.1% (43 isolates) to cefepime, 72.7% (40 isolates) to gentamicin, and 50.9% (28 isolates) to amikacin. Carbapenem resistance among isolates were as follow: 47 (85.4%) isolates showed meropenem resistance, whereas 32 (58.1%) isolates were resistant against imipenem. The 31 (56.3%) meropenem resistant isolates were also resistant to imipenem, whereas 16 (29%) meropenem resistant isolates was still sensitive or intermediate to imipenem. A total of 8 (14.5%) isolates were susceptible to both carbapenems. Most of the isolates were multi-drug resistant as they were resistant to 3 or more classes of antibiotics (Table 1).
| Isolates, No. | Pattern of Antibiotic Resistance |
|---|---|
| 1 | ATM |
| 3 | CAZ, CTX, ATM |
| 4 | CIP, CAZ, CTX, ATM |
| 1 | MEM, CIP, CAZ, CTX, ATM |
| 7 | MEM, SXT, CIP, CAZ, CTX, ATM |
| 8 | MEM, SXT, CIP, CPM, CAZ, CTX, ATM |
| 8 | GM, MEM, SXT, CIP, CPM, CAZ, CTX, ATM |
| 5 | AN, GM, MEM, SXT, CIP, CPM, CAZ, CTX, ATM |
| 4 | GM, IPM, MEM, SXT, CIP, CPM, CAZ, CTX, ATM |
| 2 | AN, IPM, MEM, SXT, CIP, CPM, CAZ, CTX, ATM |
| 12 | AN, GM, IPM, MEM, SXT, CIP, CPM, CAZ, CTX, ATM |
Abbreviations: AN, Amikacin; ATM, Aztreonam; CAZ, Ceftazidime; CIP, Ciprofloxacin; CPM, Cefepime; CTX, Cefotaxime; GM, Gentamicin; IMP; Imipenem; MEM, Meropenem, STX, Trimethoprim-Sulfamethoxazole.
4.2. Detection of Carbapenemase and Integron Genes
Among 55 A. baumannii isolates, 45 (81.81%) were positive for OXA-23, 9(16.36%) were positive for OXA-24, and 1 (1.81%) isolate was positive for OXA-58. A total of 3 isolates carried OXA-23 and OXA-24, and 1 isolate carried OXA-23 and OXA-58. The ISAba1 element upstream of blaOXA-51 was detected in 18 (32%) isolates, however, 22 (40%) isolates had an ISAba1 insertion sequence upstream of the blaOXA-23. Integron class 1 was detected in 25 (55.5%) OXA-23 carrying isolates, 2 (22.2%) in OXA-24 positive isolates, and in 1 OXA-58 carrying isolate.
4.3. Pulsed-Field Gel Electrophoresis Analysis
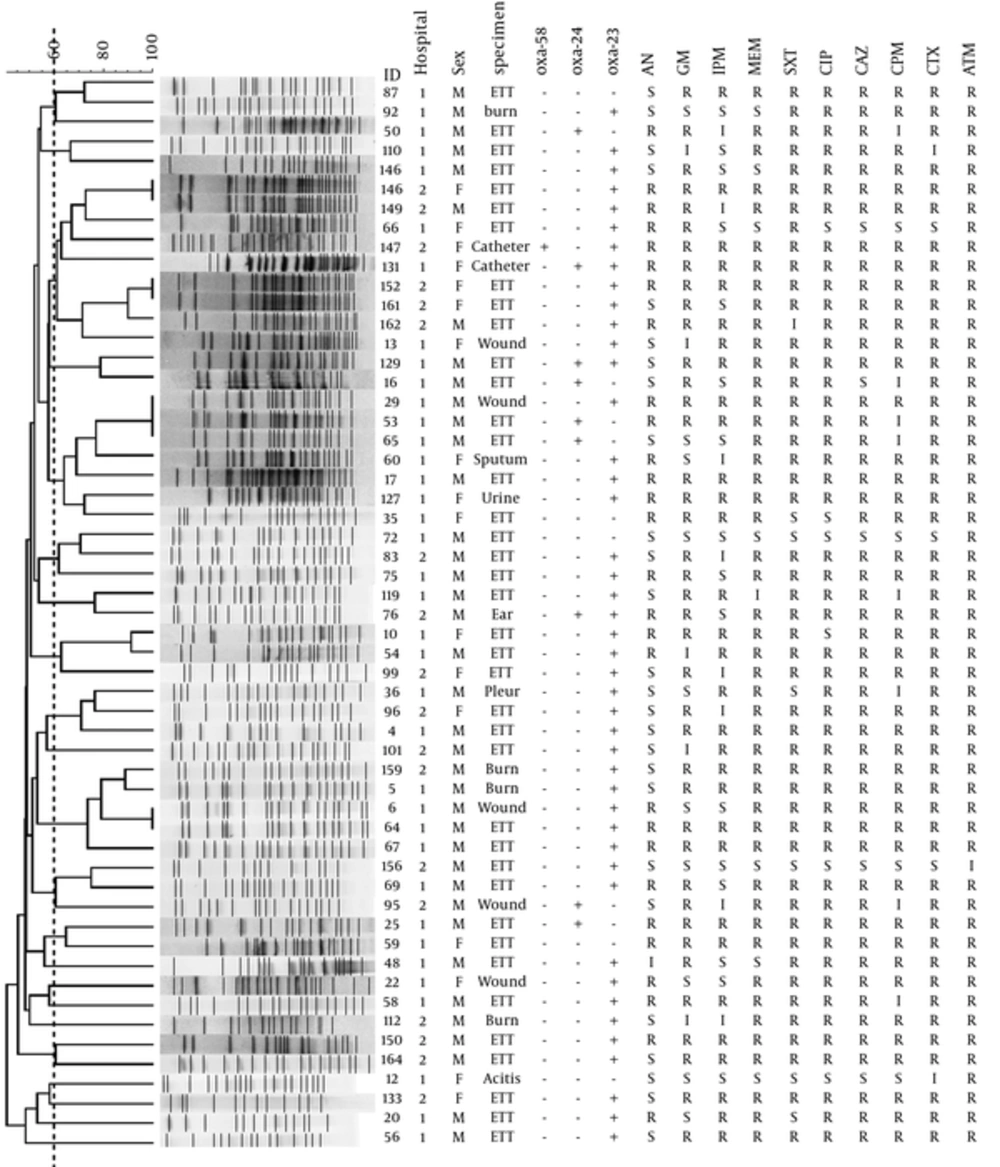
Pulsed-field gel electrophoresis analysis carried out with 55 isolates from the ICU patients in 2 hospitals resolved 50 distinct pulsotype, with the 46 single-isolate pulsotype, and the 4 multiple-isolate pulsotype. The 5 multiple-isolate pulsotype included 3 pulsotype, each accounting for 2 isolates, and 1 pulsotype with 3 isolates. The PFGE genetic similarity dendrogram was shown in Figure 1. The genetic similarity range for 55 isolates was 40% - 100%. Simpson’s diversity index was 0.996. The large cluster was not generated from the 55 isolates based on the UPGMA dendrogram. Most of the isolates were scattered throughout across the dendrogram and a few grouped as clusters.
The dotted vertical line indicates the cut-off point of 60% similarity. IMP, imipenem; MEM, meropenem; CPM, cefepime; CTX, cefotaxime; CAZ, ceftazidime; ATM, aztreonam; GM, gentamicin; CIP, ciprofloxacin; AN, amikacin; STX, trimethoprim-sulfamethoxazole; ETT, endotracheal tube; M, male; F, female; S, susceptible; I, intermediate susceptible; R, resistant.
5. Discussion
The present study reported the molecular epidemiology of carbapenem-resistant A. baumannii strains isolated in intensive care units (ICUs) patients from the 2 hospitals in Tehran. Despite a relatively low virulence of A. baumannii isolates, emerging MDR strains pose a formidable threat to patients. Acinetobacter baumannii isolation mainly occurred from respiratory specimens in ICUs patients, where they specialize in caring for the most critically ill patients and is also colonized with resistant organisms, prominently A. baumannii (14). Most of our isolates were from respiratory specimens such as the endotracheal tube. Mohajeri et al. and Shahcheraghi et al. have shown that the isolation of A. baumannii from the respiratory tract was most frequent than the other clinical samples among the ICU patients (15, 16). Although respiratory intubation is an invasive life-maintaining intervention, it is an important risk factor for A. baumannii infection in critically ill patients.
The most common definitions of MDR A. baumannii strains are resistance to carbapenems or resistance to 3 or more families of antibiotics (17). In this study, resistance to carbapenems were up to 85% in ICU isolates and the rate of MDR isolates (resistant to beta-lactams, fluoroquinolones, and aminoglycosides) were 54.54%. According to the report from Moradi et al., the prevalence of MDR isolates between 2008 and 2016 was ranged from 32.7% to 93% (18). The isolation of MDR A. baumannii in the ICU is more common and several outbreaks have been reported from Asian and European countries (8, 18-21). Antibiotic susceptibility testing in this study showed that 21.8% of patients were infected by resistant strains to all tested antibiotics. The molecular analysis of OXA genes showed that blaOXA-23 was the dominant oxacilinase in our strains. The epidemiological data in Iran show that the resistance of A. baumannii to carbapenems is mainly due to the activity of OXA-23 (5, 22, 23). The reported OXA-23 gene carrying isolates rate of A. baumannii were 52% and 85.6% in 2016, according to studies carried out in a referral hospital in the South and South-West of Iran, respectively (6, 20). OXA-23 producing isolates is considered a significant cause of A. baumannii outbreaks worldwide (24, 25).
Insertion sequences affect the expression of oxacilinase genes in A. baumannii. ISAba1 is 1180 bp insertion sequence with several copies in A. baumannii genome. Insertion of the ISAba1, an element in the upstream of oxacilinase genes, provides strong promoters for gene expression (26). The blaOXA-23, with an upstream insertion of ISAba1, was found in 40% carbapenem resistance isolates in the current study, most of those isolates were MDR isolates. In addition, the ISAba1 insertion sequence in the upstream of the blaOXA-51 was seen in 32% isolates. The results of Bahador et al. are similar to our results; however, the results of Salimizand et al. are higher (100%) than this studys’ results (27, 28).
The OXA-51-like enzyme is intrinsic in A. baumannii, the carbapenems hydrolytic activity have had studies only for OXA-51 and OXA-69 enzymes. Those 2 enzymes showed only weak carbapenems hydrolytic activity, however, the expression of the blaOXA-51-like genes increased by levels of 50-fold when ISAba1 is inserted in 7 bp upstream of the blaOXA-51-like genes; the carbapenems MICs for these isolates have been found to be similar those for acquired OXA-type carbapenemase carrying isolates (29-32). However, Pagano et al. showed that ISAba1 upstream of blaOXA-51-like was present. Also, in susceptible isolates, it was suggested that a presence of ISAba1 in the upstream of the blaOXA-51 alone is insufficient for carbapenems resistance (33). Similar results have been observed by Lin et al. in A. baumannii isolates in Taiwan. Although all ISAba1/blaOXA-51-like containing isolates in this study were carbapenems resistant, they were also co-carriage blaOXA-23 or blaOXA-24 (34).
Pulsed-field gel electrophoresis is considered the high-resolution typing methods used for local epidemiological purposes. The high Simpson’s diversity index (0.996) for endemic A. baumannii isolates in this study indicate that PFGE to be an efficient method to identify small differences between isolates within endemic clones. Pulsed-field gel electrophoresis results revealed a polyclonal distribution of our isolates in the ICUs of 2 hospitals; however, at the 60% level of similarity, 3 clusters of 4 or more isolates were observed. The remaining isolates were scattered across the PFGE dendrogram as a single or small cluster that contained less than 4 isolates. In this study, the genotype of most A. baumannii isolates unrelated to type sample, the hospital or the resistance pattern.
No significant association has been found between the pulsotype of each isolate and carbapenems resistance, MDR patterns or presence of carbapenemase genes. However, analysis of genetic relatedness and resistance genes showed that highly resistant endemic clones of A. baumannii disseminated in the ICUs of 2 hospitals. Pulsed-field gel electrophoresis analysis showed the high degree of blaOXA-23 gene mobility, where strains with same resistance gene content vary in their PFGE profile. However, a high level of similarity was obtained for ICUs isolates in the Mohajeri et al. study (35). Their isolates formed 4 clusters with 85% similarity. In the studies of Bahador et al. and Anvarinejad et al. similar to our isolates, high level of diversity was observed among the isolates (36, 37).
6. Conclusion
In conclusion, A. baumannii infection is a formidable threat to patients in intensive care units and in countries with limited resources have a high morbidity and mortality rate. The emergence of the polyclonal MDR and blaOXA-23 gene carrying A. baumannii isolates in the ICUs in this study indicate that active surveillance and health policies are urgently needed for the detection and control the dissemination of such organism.