1. Background
Candida albicans is a type of fungi that causes candidiasis in humans (1). In recent years, the prevalence of infections caused by C. albicans has increased due to antimicrobial resistance and limitations in the number of antifungal agents (2). Candida yeast may cause complications such as dysphagia, esophagitis, and urinary tract candidiasis, which can spread to other organs (3). Candida is a part of the normal vaginal flora in 20-50% of women, and therefore they do not display any signs or symptoms (4).
The prevalence of vaginal candidiasis has been reported differently in different parts of the world. A study in the United States showed that C. albicans is the most prevalent fungal species, and there is a relationship among immunosuppression, antibiotics use, and emerging C. albicans infection (5). Anoter study in Iran showed that the prevalence of vaginal candidiasis among women of the reproductive age was 67%, including symptomatic and asymptomatic women (6). Furthermore, Fatahinia et al., in Ahvaz, showed that 32% of married women had a Candida strain in direct microscopic examination and culture medium and C. albicans was the most common species (7).
Various medications such as echinocandins, caspofungin, micafungin, anidulafungin, and fluconazole are recommended for the treatment of vaginal candidiasis (8). Antifungal medications may cause some side effects in the body, including abdominal pain, diarrhea, headache, rash, indigestion, allergic reaction, and severe skin reaction (9). Some natural products are commonly used to treat C. albicans. Probiotic supplements (10), organic apple cider vinegar (11) and organic garlic (12) are examples of natural products used to prevent and treat Candida vaginitis.
Juglans nigra (black walnut) is a deciduous tree that is also referred to as eastern black walnut. This tree is cultivated in places such as the South Dakota in North America. The active component in black walnut is juglone, which can inhibit specific enzymes that are required for metabolism. Juglone is toxic to many insect herbivores and has antioxidant effects (13). The anti-cancer, anti-bacterial, antiviral, and antiparasitic effects of juglone have been previously demonstrated (14). Juglone can also tighten the skin, relieve irritation (15), and improve cardiovascular status (16). It also has antifungal and antimicrobial activity (17). More recently, Wang et al., further demonstrated the antimicrobial effects of juglone (18). Other studies have found positive effects of juglone on Pseudomonas, Burkholderia cepacia, Staphylococcus aureus, and Bacillus subtilis (19, 20). Juglone also showed antifungal activity comparable to that of other medications such as griseofulvin, clotrimazole, and tolnaftate (19). Furthermore, the antifungal activity of juglone has been demonstrated in other studies (21, 22).
2. Objectives
There is not enough information regarding the effectiveness of J. nigra green extract on C. albicans. Therefore, the main objective of this study was to compare the effects of J. nigra to those of clotrimazole on C. albicans in rats.
3. Methods
3.1. Ethics Statement
This study was approved by the ethics committee of Ahvaz Jundishapur University of Medical Sciences (Ref. No.: IR.AJUMS. REC.1394.616). We carried out a double- blind randomized controlled trial to compare the effects of J. nigra to those of clotrimazole on C. albicans in female rats.
3.2. Preparation of C. albicans Suspension
Candida albicans was isolated from a patient with Candida vaginitis (according to the DNA Data Bank of Japan, Accession number: LC201976) and used to infect rats. The cells were cultured in yeast extract peptone dextrose (YEPD) medium at 28 - 30°C. After 48 hours, the culture was centrifuged and the yeast cells were washed 3 times with sterile phosphate-buffered saline (PBS). The OD was measured[H1] using a spectrophotometer and a suspension of 1 × 108 cells/mL was prepared (1 × 107 cells in 100 µL).
3.3. Creating Vaginitis in Rats
Female Wistar rats (n = 35), with the weight of 150 - 200 grams, were purchased from the animal center of Ahvaz Jundishapur University of Medical Sciences, and were randomly divided into 5 groups (7 per group). Each group was housed in a separate cage with a sterile straw under standard temperature and light (12 hours light-dark cycle, temperature 20 - 25, and humidity 50% - 60%) for 1 week. Vaginal sample were obtained using sterile swabs dipped in PBS to ensure the absence of infection. Samples were cultured on Sabouraud dextrose agar, with and without chloramphenicol, to ensure that there was no primary infection in the rats. Candida albicans were cultured in YEPD medium. The cultured yeasts were washed with PBS 3 times after 18 hours. This mixture was centrifuged and 5 × 107 - 1 × 108 yeast cells were suspended in PBS. To suppress the immune systems of the rats, 2 doses of estradiol benzoate were injected (0.5 - 0.6 mg) subcutaneously in the lower abdominal peritoneum over 3 days. Immediately after the second injection of estradiol, 100 µL of yeast suspension was injected into the vagina and the rats were kept upside down for approximately 1 minute to ensure the inoculum.
3.4. Drug Development
Fresh Iranian walnuts with green husks (Herbarium code: JPS017113) were gathered (this type of walnut with husk is available during the fall). Green walnut husks were shade- dried and then transferred to the pharmacognosy lab at the Pharmacy School of Ahvaz Jundishapur University of Medical Sciences. The dried green husks were powdered and an extract was prepared using the maceration method. The powdered green husk was combined with 80% ethanol and the resulting solution was maintained at room temperature (27 - 32°C) for 72 hours with occasional stirring. The mixture was filtered through Whatman paper using a vacuum flask. The extract was dried using a vacuum rotary evaporator at 45°C then freeze-dried to a fine bulk powder. The powder was kept refrigerated below 4°C.
Juglans nigra green husk cream was prepared by adding the extract to a suitable cream base (emulsion of water in oil) at concentrations of 2% and 4% (w/w). In the pharmacy, 1% of clotrimazole cream was prepared using the same base. All final products had the same appearance and an approved color was used to maintain uniformity. Microbiological and physicochemical tests (uniformity, physical stability, distribution, and acidity) were performed on the prepared formulations. 2% and 4% J. nigra and clotrimazole creams were placed in similar canisters and were encoded by a blinded third party. All creams had the same appearance and texture. Creams were applied and samples were prepared by a blinded researcher.
3.5. Grouping
Rats were randomized into 5 groups:
1- A control group infected with C. albicans but not treated (positive control)
2- An un-infected control group, also not given treatment (negative control)
3- An infected group that received treatment with 2% J. nigra extract.
4- An infected group that received treatment with 4% J. nigra extract.
5- An infected group that received treatment with 1% clotrimazole.
3.6. Assessment of Infection
Four days after the first inoculation, a vaginal sample was obtained from all rats. In addition to a direct smear, the samples were cultured on Sabouraud dextrose agar with chloramphenicol (SC) (QUELAB, UK) to check for the presence of infection. An additional dose of yeast was administered to the vagina if infection was not found. According to most references, the number of yeast present in the sample is determined by the criterion of 7 - 8 colonies per swab (23). In our study, the number of colonies grown at the plate surface was counted with a colony counter machine, and more colonies of yeast were observed in a direct sample.
3.7. Treatment
After ensuring that the rats were infected with C. albicans, treatment was initiated in the different groups. A total of 2 groups received either 2% or 4% of J. nigra extract, 1 group received clotrimazole cream, and 1 group remained without treatment. One week and 2 weeks after treatment, a vaginal sample from all 5 groups obtained using a sterile swab was cultured in SC medium at 35 - 37°C. The colonies formed were counted after 48 hours.
3.8. Statistical Analysis
ANOVA was used to compare means of CFUs of C. albicans before and after intervention, and LSD post-hoc tests were utilized for detecting differences between groups. All analyses were performed using SPSS version 22. P < 0.05 was considered to indicate a statistical significance.
4. Results
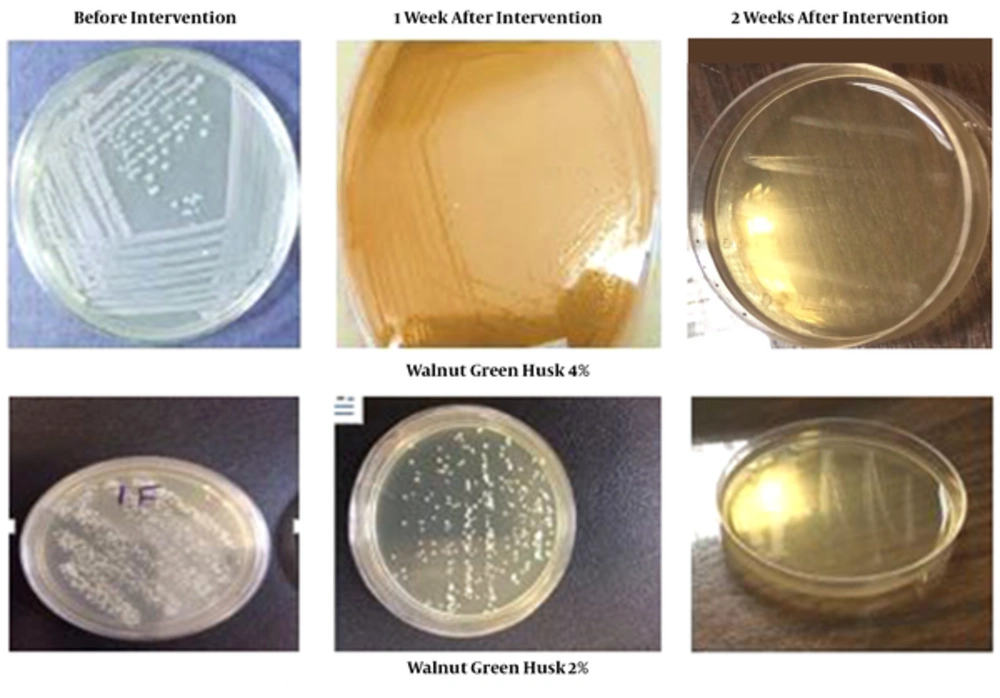
After exposure to C. albicans all rats, except for the negative control, showed colonies of C. albicans. The mean number of CFUs, before intervention, was 308.2 ± 8.73 in the 2% J. nigra group, 219 ± 13.4 in the 4% J. nigra group, 312.7 ± 28.32 in the clotrimazole group, 233.85 ± 8.15 in the positive control group, and 0 in the negative control group (P < 0.001). After 1 week, the mean number of colony forming units significantly decreased to 71.1 ± 3.9 in the 2% J. nigra group and 0 in both the 4% J. nigra as well as clotrimazole groups. The number in the positive control group remained unchanged (230.7 ± 9.86) and no CFUs were detected in the negative control group (P < 0.001). After 2 weeks, the mean number of colony forming units was 0 in all the groups, except in the positive control group (P < 0.001). The 4% J. nigra extract could eradicate colony forming unites after 1 week of treatment, whereas the 2% J. nigra extract significantly reduced the mean number of CFUs to 71.14 ± 3.97 (P < 0.001). There were no CFUs detected in either J. nigra extract treated groups after 2 weeks (Figure 1).
5. Discussion
The main objective of this study was to compare the effects of 2% and 4% J. nigra extract with those of clotrimazole on C. albicans in rats. Our results showed that the 4% J. nigra extract could eliminate C. albicans colony forming units after 1 week of treatment, which is similar to the effects of clotrimazole. The primary active component of walnut green husk is juglone. All parts of the walnut tree and its fruit have anti-oxidant effects, anticancer activity, and can boost the immune system (24). In this study we used the green husks of black walnuts.
Other studies have shown that juglone has antimicrobial and antifungal effects (25, 26). Grover et al., assessed the effect of juglone on planktonic and sessile C. albicans cells. Their results showed that at a concentration of 160 µg/mL of juglone could significantly reduce the virality of colonies of C. albicans as assessed by various tests such as XTT, AFM, confocal analysis, and SEM techniques (21). These results are in agreement with our findings. Moreover, in line with our study, Rodrigues et al., found that the green husk of J. nigra has an antifungal effect and can be used as an alternative for fluconazole against C. krusei, recurrent vaginal candidiasis, and dermatophyte infections of the nails as well as skin (27). Salamat et al., in their study, found that the extract of J. regia could prevent growth of C. albicans in fraction of 337 mg/mL (28), which is in line with our findings.
To the best of our knowledge, this is the first time that the effects of J. nigra green husk have been compared to the effects of clotrimazole on C. albicans in rats. This study has a limitation that should be mentioned. We tested clotrimazole against sensitive and resistance strains of C. albicans and then compared the activity of J. nigra extract on resistance and sensitive strains, which may have affected the results.
6. Conclusion
This study showed that J. nigra green husk in the form of a vaginal cream is effective against C. albicans in female rats. The 4% J. nigra extract was more effective than the 2% extract, and its effect was similar to that of clotrimazole. Based on our findings, further studies using J. nigra green husk vaginal creams in humans are recommended.