1. Background
The presence of fluid in the middle ear without symptoms or signs of infection is defined as otitis media with effusion (OME). Otitis media with effusion is different from acute otitis media because it is characterized by the presence of fluid in the middle ear accompanied by signs of acute inflammation of the middle ear (1). It is a disease caused by Gram-negative and Gram-positive bacterial pathogens in the middle ear of infants or children (2). In children, it is the most common cause of hearing loss and requires antibiotic therapy and surgical management. It can lead to significant side effects on hearing, and if these effects continue for a long period of time, they can cause lack of language development and speech in children (3). The mechanism of the pathogenesis of OME is not clearly understood. However, bacterial infection, Eustachian tube dysfunction, allergy and immunological factors are known as major causes for this disease.
Many studies have reported detection of various species of bacterial agents in middle ear effusion; Haemophilus influenzae, Streptococcus pneumoniae and Moraxella catarrhalis have commonly been identified. The bacterial etiology of acute OME varies in different individuals, where S. pneumoniae is the most commonly found bacteria, present in 29-32% of cultures. Moraxella catarrhalis as a pathogen in otitis media has increased during the last two decades. In studies based on PCR, especially on patients with a negative culture, Alloiococcus otitidis also was detected in a considerable proportion (4). This has resulted in the consideration of bacterial infections as an important factor in the pathogenesis of OME. The introduction of polymerase chain reactions (PCR) has resulted in a highly increased sensitivity and specificity of bacterial detection in middle ear effusion (5).
2. Objectives
Due to the prevalence of OME in children, we decided to investigate bacterial agents that cause diseases such as A. otitidis, H. influenzae, S. pneumonia and M. catarrhalis in these subjects.
3. Patients and Methods
Forty-five children between one and 15 years of age were selected for this study. The main reason for this selection was that the prevalence of this disease (OME) is highest in children. Bacteriological analysis was performed on samples taken by swabbing the middle ear cavity during a cochlear implant operation. From the patients’ medical history, particular attention was paid to the following variables: gender, age, date of onset of OME, history of conditions such as allergies, respiratory tract infection, otitis media (acute, with effusion and recurrent acute), hearing level, and unilateral or bilateral hearing impairment. Next, the external auditory canal was cleansed by 70% alcohol for one minute and a myringotomy was conducted. Middle ear fluid has high viscosity, thus an amount of 0.5 mL of normal saline was inserted into the middle ear canal and the fluid was extracted by swabbing.
3.1. Isolation of Bacteria
Seventy middle-ear samples were collected from 45 children between one and 15 years old, who had been afflicted to OME. Each specimen was divided into two portions; one portion of the extracted middle ear fluid was transmitted into micro tubes with PBS buffer for ordinary bacterial culture and the other was stored at -70°C for the PCR assay. For primary isolation of bacteria, specimens were inoculated to several culture media under aerobic conditions with 5% CO2 at 35°C for 24-72 hours according to Chapin. The following culture media were used: Muller Hinton with 5% sheep blood agar (A. otitidis and S. pneumoniae), chocolate agar with vancomycin (5 mg/mL), clindamycin (1 µg/mL) and bacitracin (300 µg/mL) for H. influenzae, chocolate agar with vancomycin (5 µg/mL), clindamycin (1 µg/mL) and bacitracin (300 µg/mL) and acetazolamide (for M. catarrhalis). Since A. otitidis has slow growth incubation was done for two weeks. Isolated bacteria were recognized by a conventional biochemical test (6-8). Thus Gram staining was performed. All media were incubated at 37°C, with the blood agar and chocolate agar plates in a microaerophilic atmosphere and Brucella agar in aerobiosis.
3.2. PCR Assay
The genomic DNA was extracted by High Pure PCR Template preparation kit (Roche, Germany). Furthermore, PCR was done with DNA extracts using universal primers (Table 1). The specific primers that were used for this study included; RDR125: ACTTTTG-GCGGTTACTCTAT and DG74: TGTGCCTAATTTACCAGDAT for H. influenza (5, 9, 10), STR1: GATCCTCTAAATGATTCTCAGGTGG and DG74: ACTATAGAAGAAAGGG-AAGTTTCCA for S. pneumonia (5, 9, 10), MCA: TTGGCTTGTGCTAAAATATC and MCAT2: GTCATCGCTATCATTCACCT for M. catarrhalis (5, 9, 10), and mer21: CTACGCA-TTTCACCGCTACAC and mer20: GGGGAAGAACACGGATAGGA for A. otitidis (11-14). Initial denaturation at 95°C for five minutes, 35 cycles of denaturation at 95°C for 30 seconds, annealing at (66°C for S. pneumoniae and A. otitidis, 55°C for M. catarrhalis and 52°C for H. influenzae) for 30 seconds, extension at 72°C for 30 seconds and final extension at 72°C for seven minutes were carried out using a DNA thermal cycler eppendorf. Electrophoresis was used for 60 minutes in 2% agarose gel for the detection of amplified products. Specimens of this study with consistent PCR results were sequenced by the Bioneer Company (Korea) and used as positive controls, while distilled water was used as a negative control.
| Bacteria | PCR +, Culture + | PCR +, Culture – |
|---|---|---|
| Alloiococcus otitidis | 1 (1.4) | 17 (24.3) |
| Haemophilus influenzae | 0 | 14 (20) |
| Streptococcus pneumoniae | 2 (2.8) | 12 (17.15) |
| Moraxella catarrhalis | 3 (4.2) | 6 (8.4) |
| Total positive (n = 55) | 6 (8.4) | 49 (70) |
a Data are presented as No. (%).
4. Results
All middle-ear samples were taken by aspiration during the surgery and were then analyzed. Based on the conventional method (culture and staining), polymorphonuclear leukocytes were not seen in any of the patients.
4. 1. Results of Culture
Of the 70 samples that were obtained from 45 children with OME, the average age of onset was 4.5 years. Of those affected, 56% were boys and 44% girls. About 20 out of 45 patients (45%) with unilateral involvement and 25 out of 45 patients (55%) with bilateral involvement had samples positive for bacterial DNA. During surgery, the quality of the effusion was analyzed visually and was mucous in 32 (71.2%) children and serous in 13 (28.8%) patients. A total of 70 samples were taken from the 45 children, 25 cases were positive for bilateral presentation; it was possible to take a sample from both ears. When analyzing bilateral samples, the samples that were obtained from both sides were 100% similar. Among the 70 samples, bacterial cultures were positive in six (13.4%) samples. The microorganisms that were isolated from cultures, included: A. otitidis, M. catarrhalis and S. pneumoniae. Haemophilus influenzae was not isolated from any of the subjects (Table 1).
4.2. Results of PCR Assay
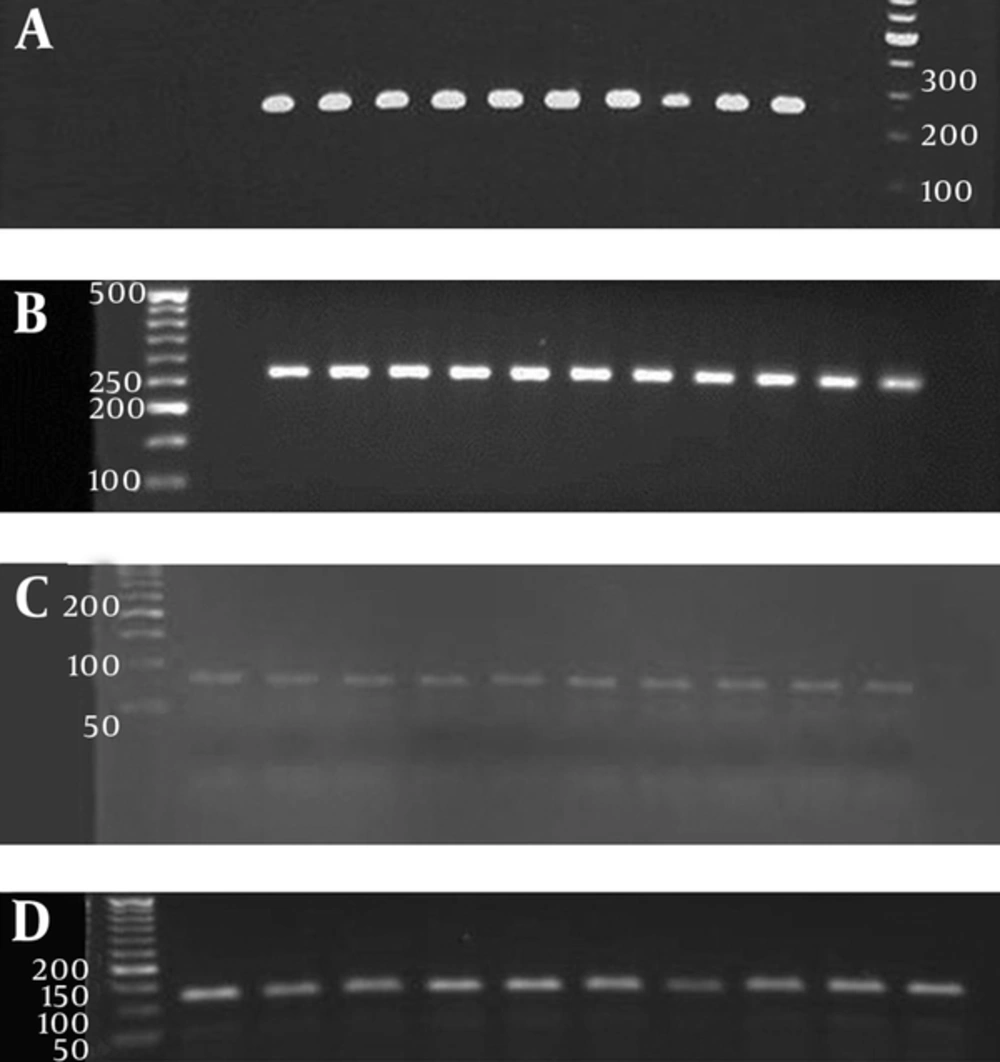
As visualized by agarose gel electrophoresis of the PCR products, A. otitidis was the bacterium most frequently isolated; there were 18 positive samples amongst a total of 55 (25.7%), and bacterial DNAs related to A. otitidis were detected in 33% and 67% of unilaterally and bilateral affected patients, respectively. Hearing levels were normal in all patients. Amongst 12 patients with bilateral OME caused by A. otitidis, unilateral infection was caused by H. influenzae and S. pneumoniae in one and two cases, respectively. The distribution of bacterial agents isolated from patients with OME based clinical data; sex and age are summarized in Table 2. The PCR assay results showed that 55 samples were positive for bacterial DNA as tested by universal primers. In these samples, A. otitidis, H. influenzae, S. pneumoniae and M. catarrhalis were detected (Figure 1).
| Variables | Alloiococcus otitidis | Haemophilus influenzae | Streptococcus pneumoniae | Moraxella catarrhalis | Total Positive |
|---|---|---|---|---|---|
| Age, > 5 y | 13 (73) | 11 (78) | 6 (43) | 3 (37) | 33 (47.1) |
| Age, ≤ 5 y | 5 (27) | 3 (22) | 8 (57) | 6 (63) | 22 (31.4) |
| Male | 12 (67) | 6 (43) | 8 (57) | 7 (75) | 33 (47.1) |
| Female | 6 (33) | 8 (57) | 6 (43) | 2 (25) | 22 (31.4) |
| Unilateral | 6 (33) | 9 (64) | 9 (64) | 6 (67) | 30 (54.6) |
| Bilateral | 12 (67) | 5 (36) | 5 (36) | 3 (33) | 25 (43.4) |
| Total samples | 18 (25.7) | 14 (20) | 14 (20) | 9 (12.8) | 55 (78.5) |
a Data are presented as No. (%).
A, 1: 50 bp DNA Ladder 2: Negative control 3: Positive control 4-13: Samples, Haemophilus influenza; B, 1: 100 bp DNA Ladder 2: Negative control 3: Positive control, 4-12: Samples, Streptococcus pneumonia; C, 1: 50 bp DNA Ladder 2: Positive control 3-11: Samples 12: Negative control, Moraxella catarrhalis; D, 1: 50 bp DNA Ladder 2: Positive control 3-11: Samples 12: Negative control.
5. Discussion
In the pathogenesis of OME, bacterial infection has been known as an important factor, and many studies have demonstrated that A. otitidis, M. catarrhalis, S. pneumoniae and H. influenzae are the most common bacterial pathogens in this infection. It has been supposed that these bacteria are included in the normal flora of the middle ear canal (15). However, many previous studies have demonstrated that some of these bacteria, such as A. otitidis have immune-stimulatory ability (16) thus could not be part of the normal flora of this tract. Detection of these bacterial agents could be performed by PCR assay and ordinary bacterial culture. However, isolation of A. otitidis via conventional culture methods is difficult because it grows slowly and requires a special medium to grow in vitro (11).
The rate of isolation of these bacteria by standard culture does not yield more than 45%. Nonetheless, when the PCR method is used the isolation rate of these bacterial agents is significantly increased and, A. otitidis is often the most common pathogen isolated (11, 16). Therefore, PCR is useful for the detection of pathogens that have slow growth rate, are difficult to culture in a diagnostic laboratory or require a special medium. Furthermore, PCR is the most functional technique for determination of the existence of pathogenic bacteria DNA in culture-sterile effusions of the middle ear. Thus these bacterial agents can easily be detected by the PCR assay (4, 16). We used both methods to study the bacterial etiology of OME in 70 children under 15 years old. The average age of onset was 4.5 years and the most common age of onset was less than five years.
In this study, using ordinary bacterial culture, bacteria were detected in only six (8.6%) cases, while using the PCR assay bacterial DNA was detected in 55 (78.6%) cases. This rate is lower than that reported by previous studies performed in Iran (17). In this study, the rate of culture positivity was 8.6% (6 out of 70 samples), while the rate of culture positivity that was reported by Khoramrooz et al. was about 47.6% (17); overall, our results were lower than other reports (4, 11, 16). M. catarrhalis was the most prevalent (4.3%) bacterial isolate among middle ear fluid samples that was detected by culture, however, our detection rate of this isolate was lower than that previously reported by Khoramrooz et al. (9.5%) (17) yet similar to reports from Lebanon (4%) (10). Furthermore, A. otitidis was isolated from 1.4% of samples while other studies from various regions have reported different isolation rates; Iran 23.8% (17), Spain 48.2% (11), Turkey 58% (18), and the United States 5% and 4.7% (7).
The third most isolated bacteria, by culture, from the middle ear fluid samples in this study, was S. pneumoniae (2.9%), which was isolated at lower rates than that reported by a similar study from Iran 9% (17), yet similar to a study from Spain 3.4% (19) and Brazil 12.5% (20). However, no H. influenzae was isolated using ordinary culture in this study. Furthermore, in this study we compared PCR with culture as a possible means of obtaining evidence of bacterial involvement in this study. According to the PCR method, which confirmed the results of the culture method, A. otitidis was the most prevalent (25.7%) bacterial isolate among the middle ear fluid samples. This isolation rate was lower than that reported by a similar study from Iran (17), but higher than that reported by some other studies (18.5%) (11) and lower than that reported from turkey 35% (18) and Japan 60.5% (13).
Using the PCR assay M. catarrhalis, S. pneumoniae and H. influenzae were detected in 12.8%, 20% and 20% of samples, respectively, and these results were somewhat similar to that reported by a study from Iran (17). The specificity and sensitivity of the PCR assay was higher than the culture method in detection of bacterial infection that caused OME. There is no reasonable description for this disagreement due to the difference in the distribution of these pathogens. In conclusion, based on this study, we accepted that A. otitidis, S. pneumoniae, H. influenzae and M. catarrhalis are major bacterial pathogens in otitis media with effusion, where A. otitidis was the most often isolated bacterium in Iranian children with middle ear effusion. Many studies have suggested that the remaining bacteria due to an inadequate use of antibiotics may be the cause of OME. Thus bacterial infection plays an important role in the progression of acute otitis media to OME, which in turn causes hearing loss, social malformation and high medical costs. This is an important factor that must be considered while treating OME in children.