1. Background
Candida albicans, a normal flora in most human, is predominantly isolated from the skin, mouth, vagina, and gastrointestinal tract. Under certain conditions such as indiscriminate consumption of antifungal drugs, immunosuppressive treatments, long-term catheterization, and longer survival of immunocompromised individuals, C. albicans changes from commensal microflora to opportunistic pathogens causing life-threatening infections (1). Resistance of albicans and non-albicans Candida spp. to antifungal drugs, especially azoles, is dramatically increasing, and therefore, further researches are needed to investigate the effect of other agents on their virulence properties. Oropharyngeal candidiasis (OPC) is the major HIV related oral lesion and about 90% of HIV+ patients develop oropharyngeal or esophagol candidiasis (2). Oropharyngeal candidiasis due to antimicrobial resistance of Candida species is a major problem for HIV+ patients (3).
The most important virulence factors of C. albicans are adhesion, dimorphism, phenotypic switching, cell wall components (beta-glucans and chitin), phospholipase production, biofilm formation, and aspartyl proteinase secretion (4). Among these factors, 10 proteins (SAP1- SAP10) usually involve in tissue invasions. Several studies confirmed that hyphal formation, adhesion, and phenotypic switching are attributed to the production of such proteins (5, 6). SAP2 is the most effective factor on the hydrolysis of many proteins, including albumin, haemoglobin, keratin, and immunoglobulins (7). An animal model study showed that SAP2 could remove yeasts and there was no sign of infection in the studies mice. Tissue analysis of the mice infected with SAP2 indicated the significant removal of C. albicans and decreased ability of yeasts for colonization and adhesion to cell surface (8).
Saccharomyces boulardii, non-pathogenic yeast, are used as probiotics in prevention and treatment of diarrhoea (9). The yeast emerges its pathogenicity through several ways such as stimulation of enzymatic activity and immune responses in the intestinal mucosa, regulation of host cell signals, and pro-inflammatory gene expression (10). Saccharomyces boulardii stimulates the intestinal mucosa by the secretion of nutritional factors and polyamines, which enhance host immune defence (11). Oral administration of live S. boulardii prevents C. albicans infection in mesenteric lymph nodes, blood, and some organs such as the liver and kidneys (12). Saccharomyces boulardii reduces inflammation and intestinal colonization of C. albicans in mice (13).
2. Objectives
The present study aimed at evaluating the impact of S. boulardii extract on SAP2 gene expression and its antifungal susceptibility on C. albicans isolates.
3. Methods
3.1. Fungal Isolates and Growth Conditions
Fourteen clinical species of C. albicans isolated from oral lesions of HIV+ patients and the standard strain ATCC 10231 were provided by mycology research center, faculty of veterinary Medicine, University of Tehran, Iran. Lyophilized S. boulardii (Biocodex laboratories) was kindly donated by Dr. Mahzonieh (Shahrekord University). Candida albicans isolates were grown on Sabouraud dextrose agar (SDA, Himedia labs, India) and S. boulardii isolates on potato dextrose agar (PDA, Himedia labs, India) both at 30°C for 48 hours.
3.2. Preparation of S. boulardii Extract
Saccharomyces boulardii extract was prepared as described in the authors’ previous paper (14) with some modifications. For this purpose, a single colony of S. boulardii was inoculated into 5 mL of 2 media cultures: 1-Yeast nitrogen base (YNB, Sigma, Germany) (pH 5.5) plus 2% D-glucose, 2- Potato dextrose broth (PDB, Ibresco, Iran); both incubated for 18 hours at 30°C. The pre-cultures were then inoculated into 250 mL YNB and PDB media and reincubated for 24 hours. One liter of the 24-h S. boulardii culture was centrifuged at 3000 × g for 10 minutes; supernatant was separated and passed through 0.22-mm filter (Sartorius, Germany) for sterilization. Then, the supernatant was extracted with one-fifth (v/v) of the solvent (ethylacetate) for 3 hours, with changing the solvent every 30 minutes. Solvent was removed by the rotary evaporator. Finally, 20- and 50-mg dry masses were obtained from S. boulardii YNB and PDB cultures. Extracted residues were normalized with methanol to the final concentration of 48 mg/mL and used as stock samples. Methanol concentration in the samples was less than 1%. Dry mass obtained from S. boulardii PDB culture was used in the experiments.
3.3. Antifungal Assay
Antifungal activity of S. boulardii extract against C. albicans isolates was assessed by those proposed in the clinical and laboratory standards institute (CLSI) M27-A3, the micro broth dilution reference method (15). Serial dilutions of the stock solution were prepared in RPMI 1640 with MOPS buffer (Sigma, St Louis, MO, USA) to get the final serial concentrations of S. boulardii extract ranging from 0.375 to 48 mg/mL. One millilitre of fungal suspension (1 - 8 × 103 cell/mL) was distributed in a 96-well plate and incubated at 30°C for 48 hours and fungal growth was then evaluated visually. Positive control comprised of broth media and fungal suspension and the negative control contained S. boulardii extract inoculated into broth media; all cultures were incubated under the same conditions. The lowest concentration that did not permit any visible fungal growth was considered as the minimum inhibitory concentration (MIC). The minimum fungicidal concentration (MFC) was the lowest concentration that did not permit any growth on the plate. The procedure was repeated three times for all the isolates.
3.4. Antifungal Susceptibility
Susceptibility of C. albicans species to antifungal drugs was assessed according to M44-A2 CLSI (16). Mueller-Hinton agar (Himedia labs, India) containing 2% glucose was used for this purpose; accordingly, a fungal suspension (106 cell/mL) was prepared. After 48 hours incubation at 30°C, the diameter of growth-inhibition zone was measured in millimetres. This process was performed before and after the treatment with S. boulardii extract. Standard antifungal disks (Rosco, Denmark) used in this experiment were: fluconazole 25 µg, ketoconazole 15 µg, itraconazole 10 µg, clotrimazole 10 µg, nystatin 50 µg, and amphotericin-B 10 µg.
3.5. Gene Expression Assay
To induce SAP2 expression, the protocol described by Wu et al., was used (17) with some modifications. Candida albicans was grown in yeast carbon base (YCB, Sigma, USA) plus 2% D-glucose and 2% bovine serum albumin (BSA, Sigma, USA) in a shaker incubator for 18 hours at 30°C; then, the culture was washed with PBS (phosphate buffer saline) to get a final concentration of 105 cell/mL. Each sample included 10 mL of C. albicans culture and 0.48 mg/mL of S. boulardii extract. All samples centrifuged at 3000 × g for 10 minutes. Total RNA was extracted from the obtained pellets using total RNeasy Mini Kit (Qiagen, Germany), according to the manufacturer’s instructions. The quantity of the extracted RNA was determined measuring the optical density (OD) at 260 and 280 nm wavelengths using a UV/VIS spectrophotometer (Ultrospec 2000, Pharmacia). The cDNA was synthesized using QuantiTect Reverse (Qiagen, Germany), according to the manufacturer’s instructions for 1 µg of total RNA.
To perform real-time polymerase chain reaction (PCR), primers were designed using the NCBI Primer-Blast (Table 1). The Act1 gene was used as the housekeeping gene. The real-time PCR was performed as described in the authors’ previous paper (8) on a real-time thermal cycler (Rotor-Gene Q: Qiagen, Germany). The reaction conditions were 95°C for 15 minutes, followed by 45 cycles at 94°C for 15 seconds and 60°C for 1 minute. The mRNA expression level of SAP2 and Act-1 was determined using QuantiTect SYBR Green PCR Kit (Qiagen). Real-time PCR was carried out in a total volume of 10 µL containing: 1 µL of cDNA, 5 µL QuantiTect SYBR Green PCR Kit (Qiagen), and 10 pM of each forward and reverse specific primer. Amplicons specificity was checked by verifying a single peak on the melting curves. All samples and controls were normalized against the reference gene. No control template and reverse transcriptase control were included in each PCR run. All assays were carried out 3 times as independent PCR runs for each cDNA sample. The ∆∆CT method (18) was used to assess the quantification.
| Primer | Primer Sequence |
|---|---|
| SAP2 (FW) | TGCTCTTGCTATTGCTTTATTAGTC |
| SAP2 (REV) | TAGTAACTGGGAATGCTTTAGGAG |
| Act1 (FW) | CTTCACTGCCTTATCTAATTGTTCA |
| Act1 (REV) | GCTGTCCAACTTTGATGAGTTCC |
3.6. Statistical Analysis
Statistical analysis was performed using SSPS version 19; P < 0.05. Paired and one-sample Student t tests were used to examine significant differences between the groups.
4. Results
Saccharomyces boulardii extract was prepared on 2 media cultures: YNB plus 2% D-glucose, and PDB. The total dry mass obtained from 1 litre of YNB culture was 20 mg, whereas it was 50 mg from PDB culture. Saccharomyces boulardii extract did not show any fungicidal and inhibitory effects against C. albicans isolates. Antifungal susceptibility of C. albicans to ketoconazole and itraconazole changed after treatment with S. boulardii extract. Before the exposure of C. albicans isolates to S. boulardii extract, %53.3 of the isolates were sensitive to ketoconazole, whereas it increased to %73.3 after the exposure to ketoconazole. The rate of sensitivity to itraconazole increased from %6.7 to %26.7 of after treatment (Table 2).
| Susceptibility Pattern | Before Exposure (%) | After Exposure (%) |
|---|---|---|
| Ketoconazole | ||
| Sensitive | 53.3 | 73.3 |
| Intermediate | 33.3 | 26.7 |
| Resistant | 13.3 | 0 |
| Itraconazole | ||
| Sensitive | 6.7 | 26.7 |
| Intermediate | 86.7 | 73.3 |
| Resistant | 6.7 | 0 |
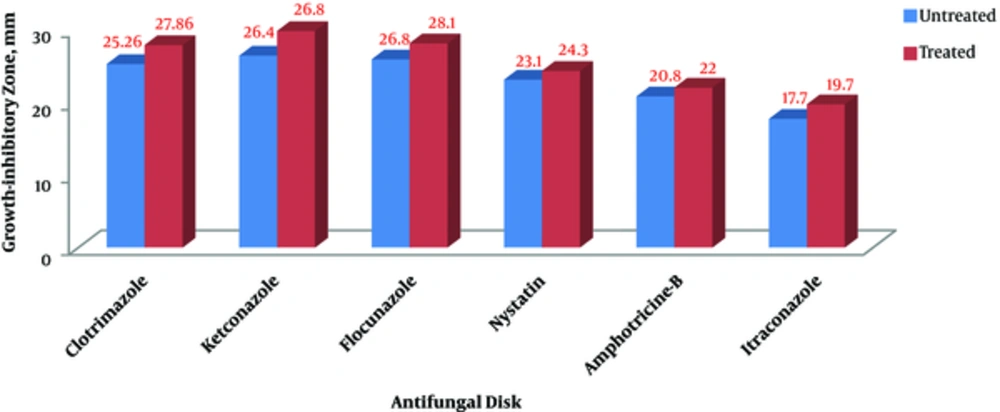
Although susceptibility pattern of C. albicans isolates to other antibiotics (fluconazole, clotrimazole, nystatin, and amphotericin-B) did not change after exposure to S. boulardii extract, their antifungal inhibitory zones showed a significant difference before and after the exposure to the extract. The mean size of the growth-inhibitory zone in the treated isolates was significantly higher than that of the untreated isolates (Figure 1 and Table 3).
| Pair (Before and After Treatment with S. boulardii Extract) | Mean (mm) | Std. Error Mean | Sig. |
|---|---|---|---|
| Clotrimazole | 0.60000 | 0.001 | |
| Before | 25.2 | ||
| After | 727.86 | ||
| Ketoconazole | 1.09022 | 0.008 | |
| Before | 26.4 | ||
| After | 29.8 | ||
| Itraconazole | 0.60945 | 0.005 | |
| Before | 17.7 | ||
| After | 19.7 | ||
| Fluconazole | 0.65611 | 0.005 | |
| Before | 25.9 | ||
| After | 28.1 | ||
| Nystatin | 0.36775 | 0.006 | |
| Before | 23.1 | ||
| After | 24.3 | ||
| Amphotericin-B | 0.36775 | 0.006 | |
| Before | 20.8 | ||
| After | 22 |
aA significant difference was observed between the mean sizes of growth inhibitory zone in treated and untreated C. albicans isolates. Statistical analysis was performed using the paired samples t test. P values < 0.05 were considered significant.
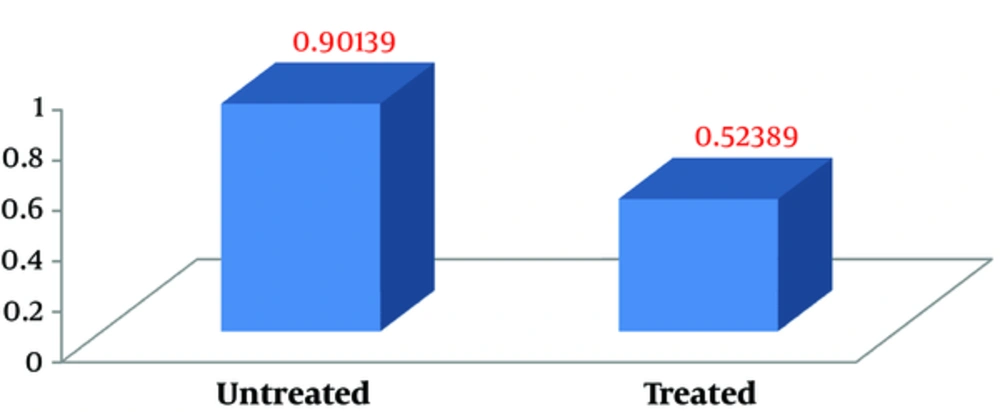
To determine the MIC of the extract influenced by SAP2 gene expression, 0.48 - 0.24 and 0.16 mg/mL concentrations were examined. SAP2 gene expression did not show any changes in 0.16 mg/mL. SAP2 gene expression also showed no significant reduction in the treated isolates compared with the untreated ones in 0.24 mg/mL of the extract. In 0.48 mg/mL S. boulardii extract, the SAP2 expression level was significantly downregulated (P < 0.009) (Figure 2).
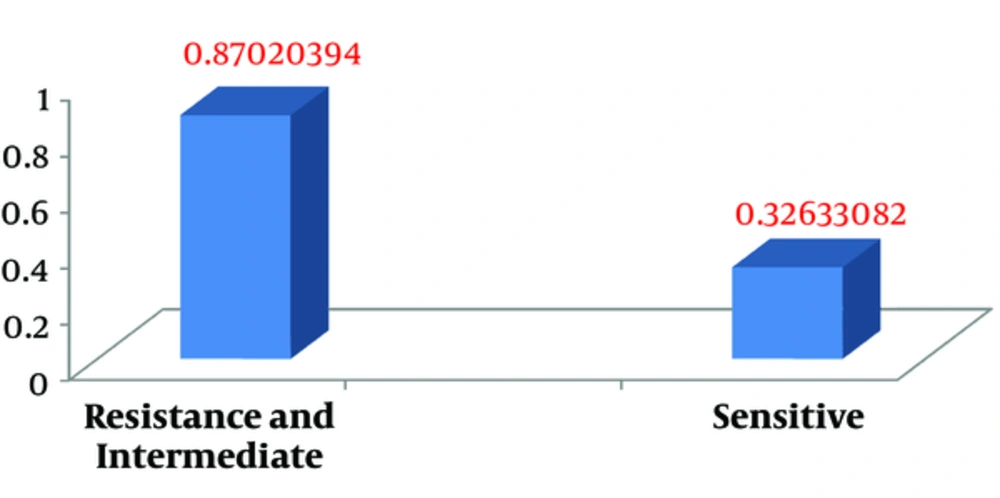
Independent samples t test used to compare the mean values of SAP2 gene expression in ketoconazole-resistant, -intermediate, and -sensitive C. albicans isolates demonstrated that the SAP2 gene expression level in resistant and intermediate isolates was significantly higher than that of sensitive isolates (P < 0.02) (Figure 3 and Table 4).
| Susceptibility Pattern | No. | Std. Error Mean | Mean | Sig. |
|---|---|---|---|---|
| Resistance and intermediate species | 7 | 0.177166733 | 0.87020394 | 0.020 |
| Sensitive species | 8 | 0.71790599 | 0.32633082 |
aStatistical analysis was performed using independent samples t test. P values < 0.05 were considered significant.
5. Discussion
Resistance of C. albicans against antifungal agents is dramatically increasing in recent decades due to indiscriminate consumption of antimicrobial agents in order to prevent and treat infections. SAP2, an important potential virulence factor of C. albicans, is a suitable target for a new research. The current study aimed at investigating SAP2 gene expression and antifungal susceptibility patterns of C. albicans species isolated from oral lesion of HIV+ patients and C. albicans standard strain ATCC10231 before and after treatment with S. boulardii extract. Dry mass obtained from 1 litre of YNB culture was 20 mg, whereas it was 50 g for the PDB culture. The current study achievements were higher than previous reports by 25 - 35 mg dry mass from 2 litres of YNB culture (14). Saccharomyces boulardii extract did not show any fungicidal and inhibitory effects against C. albicans isolates.
Results of the current study were similar to those of the previous ones; confirming the potential ability of S. boulardii extract on growth restriction and removal of C. albicans (14, 19). Dry masses obtained from PDB and YNB cultures and used in the current study experiments showed similar results. Antifungal susceptibility pattern of C. albicans to ketoconazole and itraconazole changed after treatment with S. boulardii extract. The SAP2 relative expression level was significantly downregulated after the exposure to 0.48 mg/mL of S. boulardii extract. Also, SAP2 gene expression level in resistance and intermediate isolates was significantly higher than that of the sensitive isolates. The results are similar to those of previous studies.
Wenli Feng et al., found that SAP2 activity of itraconazole-resistant C. albicans strains was higher than that of itraconazole-sensitive strains. They measured relative expression levels of CDR1, CDR2, MDR1, and SAP2 using real-time PCR. Positive correlation between relative expression of SAP2 and MDR1 was demonstrated (20). Anna Murzyn et al., reported that the expression level of HWP1, INO1, and CSH1 (genes associated with C. albicans virulence) decreased in the isolates treated with S. boulardii extract (21). Anna Krasowska et al., demonstrated the inhibitory effect of live S. boulardii and its extract on the hyphal and pseudohyphal formation (14). Results obtained from the current and previous researches indicated that S. boulardii extract can inhibit some virulence factors such as SAP2, and thereby, change antifungal susceptibility of C. albicans.
6. Conclusion
In conclusion, since S. boulardii extract did not show any fungicidal and inhibitory effects against C. albicans, it can be considered as a suitable probiotic candidate to control and treat C. albicans infections.