1. Background
Zoonotic cutaneous leishmaniasis (ZCL) is polymorphic disease with various clinical manifestations (1, 2). Leishmaniasis, a group of parasitic infections, has a worldwide distribution (3); the world health organization estimates a prevalence of approximately 12 million cases, with an annual mortality rate of 60,000 and an at-risk population of approximately 350 million (4). Approximately 90% of CL cases occur in just seven countries, including Iran (5). Several molecular targets for diagnostic PCR testing have been evaluated in Leishmania, including minicircle kinetoplast DNA (kDNA) (5, 6), the mini-exon (spliced leader RNA) gene (7), gp63 polymerase chain reaction analysis of restriction fragment length polymorphism (PCR-RFLP) (8), and the internal transcribed spacer (ITS) (1, 3, 9, 10). PCR is a new alternative to existing diagnostic procedures, such as direct smear of parasites from clinical specimens or by cultivation, with microscopic examination.
2. Objectives
In the present study, as described previously (1, 11, 12), we used the RFLP analysis of amplified ITS1 in ribosomal operons to investigate the parasite that causes ZCL, and the genetic variations among L. major isolates from Chabahar, a port city in Southeast Iran (situated at the Iran-Pakistan border). In this region, the manifestation of ZCL as a new focus of disease was in doubt, but our findings showed that the species is L. major with variable genes. The disease was correlated with the clinical manifestations of ZCL in Chabahar, so this is a new major public health problem.
3. Materials and Methods
3.1. Study Area and Population
A variety of nucleic acid detection methods that target both DNA and RNA have been developed (1). The ITS1-RFLP PCR assay is a multipurpose tool for the diagnosis of Leishmania from clinical samples, and it enables determination of the infecting species (3). The procedure was performed as described by Schonian et al. The study was approved by the institutional ethics committee, and all patients signed informed consent forms.
We evaluated the PCR-RFLP assay of the ITS1 genes for direct identification of Leishmania species in 24 out of 33 suspected patients. These patients were referred for diagnosis to the central laboratory of Chabahar (25° 17’ N.70° 38’E; 13,162 km2) for evaluation of skin diseases. The average annual temperature and humidity in Chabahar are 36.4°C and 75.9%, respectively. The population migration into and emigration out of this port have important medical implications.
The clinical samples were taken from patients suspected to have CL in different endemic areas of Chabahar, including Negor and other districts and villages. Each diagnosis was confirmed by the demonstration of amastigotes on the slit smear and/or the presence of flagellated promastigotes in Novy-MacNeal-Nicolle (NNN) medium from skin samples of 24 patients with typical and atypical lesions.
For the parasitological investigation, three skin samples were taken from the edges of lesions using sterile surgical blades. Two were used for direct smears and were stained with giemsa, and the third was inoculated into sterile screw-tap tubes that contained blood agar slanted in NNN medium, then incubated at 25 ± 1°C. The isolated promastigotes were subcultured in RPMI-1640 medium supplemented with 15% fetal bovine serum (FBS), 100 μg/mL streptomycin, and 100 U/mL penicillin. Finally, 24 positive-culture isolates were selected for molecular examination.
3.2. Parasite Samples
The diagnosis of leishmaniasis was confirmed by the presence of amastigotes in slit smears and/or the presence of flagellated promastigotes in NNN medium from typical and atypical lesions. For parasitological investigations, three skin samples were collected from the edges of the lesions using sterile surgical blades. Two of the samples were used for direct smears and were stained with giemsa, and the third was inoculated into sterile screw-top tubes containing blood agar slanted in NNN medium, then incubated at 25 ± 1°C. The isolated promastigotes were subcultured in RPMI-1640 medium supplemented with 15% FBS. They were washed twice by resuspension in buffered saline, then centrifuged. The pellets were stored at -70°C until further processing.
Parasites from a 15 mL mid-logarithmic phase of the bulk culture were harvested by centrifugation (700 × g for 20 min at 4°C) and washed three times in ice-cold sterile PBS (pH 7.2).
3.3. DNA Preparation
For species identification, the DNA isolated from amastigotes in ulcers and promastigotes in cultures was extracted using a commercial extraction kit (High Pure Template DNA Preparation Kit, Roche, Germany), according to the manufacturer’s instructions.
Leishmania reference strains were obtained from Isfahan and Tehran universities. The target ITS1-DNA was amplified in a reaction mixture that consisted of 1 μL of LITSR: 5’-CTTG GATCATTTTCCGATG-3’ and 1 μL of L5.8S 5’-TGA TAC CAC TTA TCG CAT T-3’ (12). The reaction was carried out with the PCR-Ready Supreme mix in 47 μL of total reaction, 15 μL of deionized water, 5 μL of target DNA, and 25 μL of master mix. Amplification products were separated in 1% agar gel and visualized under ultraviolet light after staining with ethidium bromide.
Leishmania. major (MHOM/IR/15/ER) and L. tropica (MHM/SU/79/K27) (13) were used as positive controls. Distilled water instead of the DNA template was used as the negative control. The designed program for Leishmania DNA amplification was an initial denaturation cycle at 95°C for 5 minutes; 30 cycles with cycle 1 at 95°C for 20 seconds (denaturation), cycle 2 at 53°C for 30 seconds (annealing), cycle 3 at 72°C for 1 minute (extension), and a final extension at 72°C for 7 minutes.
3.4. Restriction Fragment Length Polymorphism
The PCR products from ITS1-DNA PCR were digested using the HaeIII restriction endonuclease enzyme and the Taq1 restriction enzyme, as recommended by the manufacture (New England BioLabs, Inc.). The ITS1 sequence was amplified from all isolates, and reference stains were digested separately with restriction enzymes, according to the manufacturer’s instructions.
4. Results
We used the PCR-RFLP assay of ITS1 genes for the direct identification of Leishmania species in 24 out of 33 patients suspected to have CL. These 24 patients, who were positive on smears and cultures for L. donovani bodies demonstrated microscopically, were selected for the study. The patient characteristics are shown in Table 1, including gender, age, number of lesions, ulcer duration, and distribution of ulcers on the body.
| Data Samples | Age, y | Gender | Number of Ulcers | Ulcer duration, m | Ulcer Location | Leishmania spp. | Genotype Pattern |
|---|---|---|---|---|---|---|---|
| 1 | 9 | M | 2 | 2 | Hand | L. major | LmA |
| 2 | 12 | M | 1 | 1 | Leg | L. major | LmA |
| 3 | 8 | M | 3 | 3 | Hand and leg | L. major | LmA |
| 4 | 5 | F | 3 | 2 | Hand and leg | L. major | LmA |
| 5 | 10 | M | 4 | 3 | Hand, leg, and trunk | L. major | LmA |
| 6 | 15 | F | 3 | 2 | Leg | L. major | LmA |
| 7 | 18 | M | 2 | 2 | Leg | L. major | LmA |
| 8 | 8 | F | 2 | 2 | Neck | Flagellates | - |
| 9 | 25 | M | 3 | 3 | Hand and face | L. major | LmA |
| 10 | 6 | M | 1 | 1 | Hand | Flagellates | - |
| 11 | 23 | F | 2 | 2 | Hand | L. major | LmA |
| 12 | 17 | F | 1 | 3 | Hand | L. major | LmA |
| 13 | 9 | M | 2 | 2 | Hand and leg | L. major | LmA |
| 14 | 17 | M | 2 | 2 | Hand and face | L. major | LmA |
| 15 | 29 | F | 2 | 4 | Hand | L. major | LmA |
| 16 | 7 | M | 3 | 2 | Hand, leg, and trunk | L. major | LmA |
| 17 | 21 | M | 2 | 4 | Hand | L. major | LmA |
| 18 | 12 | F | 2 | 1 | Hand | L. major | LmB |
| 19 | 9 | M | 4 | 2 | Hand and leg | L. major | LmA |
| 20 | 15 | M | 2 | 3 | Leg | Crithidia | - |
| 21 | 6 | F | 1 | 1 | Hand | L. major | LmB |
| 22 | 28 | M | 3 | 2 | Hand | L. major | LmA |
| 23 | 17 | M | 2 | 2 | Hand and leg | L. major | LmA |
| 24 | 7 | M | 3 | 2 | Face | L. major | LmA |
Main Characteristics of 24 CL-Positive Patients
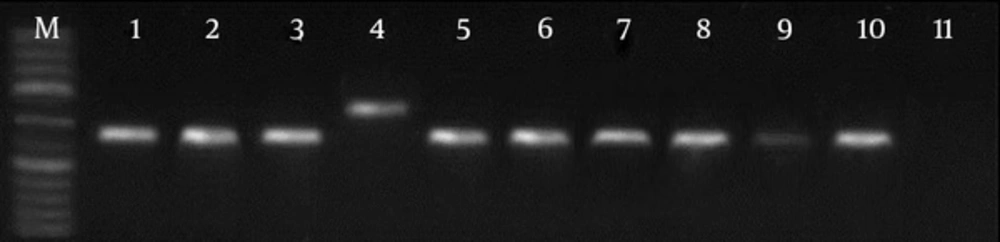
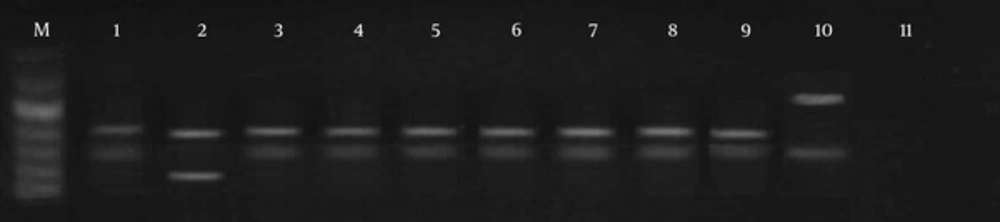
With the use of LITSR/L5.8S primer for PCR amplification, single products of the expected size of 350 bp of L. major were detected in 21 patients (Figure 1). In three isolates, a nonspecific 450 bp band was seen. RFLP was performed on the ITS1 region in the ribosomal operons of 24 isolates of L. major. After using the restriction enzyme HaeIII, banding patterns, including fragments of 140 bp and 210 bp bands, were observed in 19 cases (LmA). In three cases with this enzyme, 300 bp and 150 bp bands (Figure 2) were observed, probably flagellates. No digestion was observed in two cases.
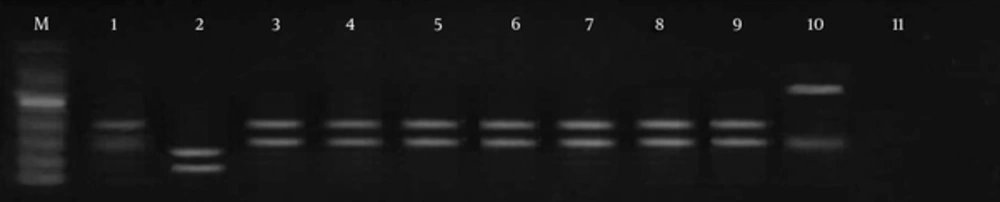
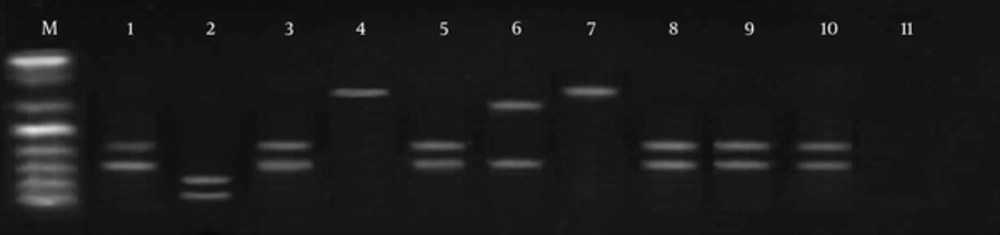
Digestion of ITS1 from ribosomal DNA with Taq1 enzymes enabled us to identify two different patterns. Using the Taq1 restriction enzyme, banding patterns including 150 bp and 200 bp were observed in 19 cases, same as the standard of L. major (genotype pattern LmA). In three isolates, digestion with this enzyme showed 300 bp and 150 bp banding patterns, dissimilar to the L. major reference strain. In two cases, no digestion was observed (LmB) (Figures 3 and 4).
M, molecular marker (50 bp); lane 1, standard L. major; lane 2, L. tropica; lane 3, PCR products; lane 4, PCR products; lane 5, PCR products; lane 6, the only sample different from the normal samples and standard patterns, probably flagellates; lane 7, PCR products; lanes 8 - 10, PCR products; lane 11, negative control.
5. Discussion
Leishmaniasis is a major societal health problem that mostly affects populations where essential health services are not easily accessible and the detection of CL is based on clinical characteristics (14). The present study shows that CL is caused by L. major in Chabahar, Iran. Parasitological methods are the gold standard for the diagnosis of CL, with a high dependence on the number of parasites in the samples and a requirement of technical skill for sampling. The sensitivity of these methods varies from 27% to 85% for the diagnosis of leishmaniasis, which is their main disadvantage (15). In addition, contamination of the cultured medium is an occasional problem; such material must be discarded.
Molecular techniques have proved to be sensitive and powerful tools for detecting Leishmania directly in clinical samples, as well as for parasite characterization using PCR (1). When PCR is appropriately applied, genetic information can be obtained (16). Some studies on Viannia isolates from different hosts and geographic areas have found high levels of intra- and interspecific variation. Schonian et al. established a diagnostic ITS1 PCR-RFLP method using the restriction enzyme HaeIII for leishmaniasis, combining high sensitivity for detecting Leishmania directly in clinical materials and the ability to identify all medically relevant species groups (12). In our study, using the HaeIII restriction enzyme, the restriction analysis of the amplified ITS1 ribosomal DNA revealed two different schizodeme patterns (LmA and LmB) in L. major.
On the other hand, some researchers have found that HaeIII was not sufficient to distinguish all species of Viannia (16, 17). We applied more restriction enzymes in the diagnosis of Leishmania species using ITS1-RFLP PCR. We found that HaeIII, Rsa, and Hinf1 can produce two profile bandings; Alu1 produces three; Dde1 and Scrf1 produce four; and Taq1 produces five (1). In this study, similar to the studies of de Almeida et al. (18), Doudi et al. (19), and Mirzaie et al. (20), two profile bandings (220 bp and 140 bp) in HaeIII digestion were detected, similar to the standard. The most prevalent genotypes related to isolates in Chabahar were LmA. Taq1 digestion showed two patterns with 150 bp and 200 bp bands. In contrary, in a study in Yazd (20) on molecular identification using Taq1 restriction enzymes, four patterns were produced. This situation must be related to special genotypes in some regions. It should be emphasized that leishmaniasis in Chabahar is new, and genetic variation is limited.
The results of the present study showed that CL infections were more common in males (48.4%) than in females (24.2%) (Table 2), probably because males spend more time outdoors than females do in this region. Our results showed relatively good concordance between parasitological results, cultures, and PCR. Some studies have shown that PCR methods achieved positive results in more than 90% of CL cases (13). The Crithidias are able to endosymbiotically live within some parasites related to the Trypanosomatidae family and sub-families of Leishmania, and move to different hosts along with them. Some researchers believe that the only way to isolate Crithidia from Leishmania is through kDNA and/or ribosomal RNA genes. It has been suggested that by extracting kDNA and/or rDNA, then amplifying them with PCR using the PCR-RFLP technique with the HaeIII enzyme and isolation of bands at 350 bp for Leishmania and at 450 bp for Crithidia, these two parasites can be isolated from each other (21). However, ITS1-RFLP PCR showed good sensitivity, and two ITS1 analyses showed the same results, as in a recent study by Doudi et al. (21).
| Samples | Female, No. (%) | Male, No. (%) |
|---|---|---|
| Positive | 8 (24.2) | 14 (48.4) |
| Negative | 2 (6.06) | 7 (21.2) |
| Total | 10 (30.26) | 23 (69.6) |
PCR Diagnostic Ratio of CL in Chabahar According to Sex
Thus, the results of the present study confirm that in the border area of Sistan Va Baluchestan, on the peninsula of Chabahar, L. major is the causative agent of CL, with genotypic variation in this area.