1. Context
Measles is a common contagious disease can cause important public health problem worldwide. The ancient Iranian scientist, Mohammad Ibn Zakaria AlRazi, is the first who differentiated between smallpox and measles in the ninth century, AD (1). Measles is an infectious disease caused by an RNA virus from the genus Morbillivirus in the Paramyxoviridae family that easily spreads by air and causes severe clinical symptoms. It is characterized by high fever (up to 105°F), malaise, cough, coryza, and conjunctivitis (the three “C”s), and a pathognomonic exanthema (Koplik spots) followed by a maculopapular rash. The rash usually appears approximately 14 days after exposure; however, the incubation period ranges from 7 to 21 days. The rash spreads from the head to the torso and finally to the lower extremities. Patients are contagious from 4 days before to 4 days after the rash appears. Measles is caused by a single-stranded, enveloped RNA virus with 1 serotype. Humans are the natural host for the measles virus. Common complications include otitis media, bronchopneumonia, laryngotracheobronchitis, and diarrhoea. Measles can cause a serious illness requiring hospitalization (2).
One in 1,000 measles cases may develop sequelae such as encephalitis, which can result in constant brain damage. One to two in 1,000 children who become infected will die from measles complications. Subacute sclerosing panencephalitis (SSPE) is an uncommon delayed complication that results in a degenerative disease of the central nervous system (CNS) which characterized by intellectual deterioration that develops a few years after the measles infection. Subacute sclerosing panencephalitis incidence decreased significantly after the measles vaccine was developed (2). Isolation of the measles virus in 1954 by Enders and Peebles led to the development of the first measles vaccines in the early 1960s (3), and in 1963, the Edmonston B strain was licensed in the United States. Two additional live attenuated measles vaccine were derived from the Edmonston strain: the Schwartz strain in 1965 and the Moraten strain in 1968. In 1973, the AIK-C strains (2, 3) became available for measles vaccine production Three other attenuated measles vaccines used internationally are unrelated to the Edmonston strain and are produced from locally derived wild-type measles virus strains including the Leningrad-16 strain (Russian Federation), the Shanghai-191 strain (People’s Republic of China), and CAM-70 (3) (Table 1).
2. Evidence Acquisition
The current study was carried out to aim measles vaccine manufacturing and consequences of that in Iran, using various search engines. Almost complete search conducted in medical databases including PubMed, Scopus, Web of Science, Scientific Information Database, Iran Medex, Magiran and Google Scholar.
3. Results
3.1. Measles Epidemiology, Pathogenicity and Complication in the Pre-Vaccination Era, Iran
In the period before the measles vaccine was licensed in 1963, an average of 150,000 - 500,000 measles cases and 10,000 measles deaths were reported in Iran annually. Although it is likely that most cases of measles were unreported, (4). In the early 1980s, the pre-vaccine era mortality rate from measles was 10 - 15%. Most of these cases were in children under 2 years old. Due to the lack of efficient and accessible health care services throughout Iran, many cases went unreported, and patients passed through the disease period at home. Before 1983, measles vaccination were not routine in the public health sector. Because of low socioeconomic status (SES) and poor health, particularly in low income families, disorders such as malnutrition and vitamin A deficiency led to the marked severity of measles in Iran and other developing countries (5).
The annually reported cases included progressive chronic disability from SSPE and acute encephalitis in many people with the disease. Measles strains were isolated and maintained by co-cultivation of infected brain cells with fresh Vero cells. The biological characteristics of the isolated strains showed that these strains remained cell-associated after repeated co-cultivations with Vero cells and that they produced plaques under fluid medium or traga-canth overlay (5). The data indicated the existence of the viral (measles) antigen and antibody in the salivary glands of all SSPE patients in Iran. Unusual clinical and laboratory features among these patients were the occurrence of chorioretinitis in 25% and papilledema in 10% of the patients. The coexistence of cerebellar cystic astrocytoma with SSPE was described in one SSPE patient (6).
These complications had a relatively high incidence in Iran. The results of a 10-year study on SSPE found that more than 66.6% of 424 measles patients were 7 to 9-year old children who were diagnosed as SSPE-positive from just one hospital between 1975 and 1984 (Figure 1). The SSPE gender distribution was 76% male and 24% female (7).
3.2. Available Vaccines and Vaccination Campaigns in Iran
The first measles vaccination was administered using three imported vaccines in 1967 (4). A comparative study of the clinical information for these vaccines indicated that there were more severe reactions from the attenuated measles vaccine than from the further attenuated vaccines and there was a high conversion rate for Edmonston B, Schwartz and Bikenham-31 vaccines in rural areas of Iran during the first measles immunization program. Two other measles vaccination strains, “Denken” and “Biken”, were designed to evaluate clinical and serological reactions to explore the possibility of their large-scale use in Iran (8). Both vaccines resulted in serological responses showing that they were highly effective. However, rashes appeared in 26.5% of patients who received the Denken vaccine, compared with 8.7% of patients who received the Biken vaccine (8).
The first local production of the live attenuated measles vaccine began in 1967 at the Razi Institute using the Sugiyama strain, a Japanese measles strain adapted and attenuated with new-born calf kidney cells (CK) (9). In two separate field trials (10, 11) the vaccine was found to be safe and highly immunogenic, inducing a seroconversion of approximately 95% in susceptible children. By the end of 1971, the number of vaccines in rural Iran was more than 3.5 million, which accounted for almost 37% of the susceptible age group (11). By the end of 1972, approximately 5 million children received this vaccine. This vaccine progressively decreased the incidence of measles and largely reduced infant mortality (12, 13). Another more attenuated strain, Sugiyama or 5F100, was cloned in AIPo4 and passaged in CK cells to use for the large-scale production of live measles vaccines (14). This new vaccine was more attenuated than the previous one. Because of its lower thermal reaction, short sporadic rash duration, and high level of protection with low production costs, it was largely produced in Razi and used in the mass immunization program. By the end of 1975, over 7.5 million children from 9 months to 5 years of age were vaccinated in Iran.
By the large-scale production and application of more than six million vaccines at the Razi institute, the measles epidemic and its associated paediatric problems were drastically reduced (11). From 1967 to 1976, the Razi institute produced more than 12,000,000 live attenuated lyophilized measles vaccines, using a highly attenuated Sugiyama strain, for mass vaccinations in south and west Iran. The vaccine’s effectiveness on the measles epidemic conferred more than 50% immunity to more than 12,000,000 vaccinated children during this era (12). Although hyperattenuation of the Sugiyama strain provided a new and efficient vaccine in Iran, it was often impossible to determine if a resulting widespread rash was caused by the vaccine or a modified case of measles. Clinical reactions following inoculation with this vaccine were common, and some physicians were reluctant to continue using it. Therefore, Razi decided to the change cellular substrate and viral strain.
3.3. Development of a New Measles Vaccine in Iran
The successful development of a measles vaccine using viral strains developed by Makino et al., in 1973 at the Kitasato Institute in Japan with cooperation of IRAN (Razi) (14) marked the beginning of research that made available the new live attenuated measles vaccine (MV), AIK-C. The strain was named AIK to represent the three countries that collaborated to develop it. The “A” represents America because the Edmonston strain became licensed in the United States as the first live isolate of measles virus (MV) in 1963. The “I” represents Iran for the Iranian researcher, Dr. Nazari’s, idea to use sheep fibroblasts. Finally, the “K” represents the Kitasato Institute in Japan, where the viral strain was developed. This live attenuated vaccine became licensed for production in Japan. AIK-C was manufactured by being cultured, adapted and cloned in chicken embryos derived from AIK, and it achieved excellent serological and epidemiological results in Japan when administered to children aged 8 months to 8 years (15).
3.2.1. AIK-C/HDC: An Iranian Measles Vaccine Strain
Due to the development of the live attenuated measles vaccine by Enders and his colleagues in 1960 (1), Iran was among the countries that launched mass vaccination campaigns against measles in 1967. Razi was the only centre for production of the measles vaccine, but it had difficulty obtaining specific pathogen free (SPF) eggs because of sanction and the cost of importation of (SPF) eggs as well. This substrate was not readily available, and when eggs were imported, the cost per dose was prohibited. Heterogeneity and viral contamination of the non-human primate cell cultures caused additional problems in producing the viral vaccine. There was an urgent need for production of large-scale viral vaccines that were readily available by establishing a “cell seed system”.
The stability and integrity of the human diploid cell strain, MRC-5, was established by Jacobs (16) and widely used as an alternative cell substrate for producing viral vaccines. This new cell system approach to producing the measles virus vaccine was widely accepted, and allowed for the safe and effective large-scale production of different human viral vaccines at Razi. Development of human diploid cell MRC-5 stock in 1960 was largely used to produce the Sabin polio vaccine (tOPV types I, II, III) by the Razi institute (17) and was essential for changing the cell substrate from CHEF to MRC-5 to develop the new measles vaccine strain. AIK-C was adopted to the human diploid cells (HDC), MRC-5, at the Razi Institute, and named AIK-C/HDC. This new measles strain was evaluated in a comparative field trial in which five different measles vaccine strains adapted to MRC-5 cell were tested (18).
3.2.2. Production and Comparative Study of AIK-C/HDC (MRC-5) Measles Vaccine
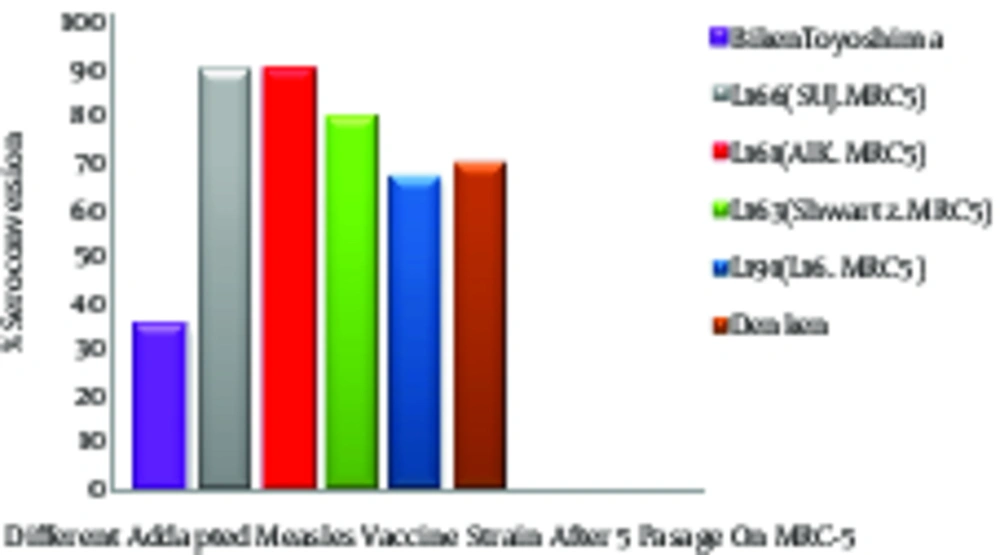
After adapting the AIK-C to MRC-5, the results of the new vaccine were compared with four other experimental measles vaccines had been adopted and produced in human diploid cells, Biken-CAM, Leningrad-16, Schwarz, and Sugiyama SF100. The purpose of this experiment was to ensure that changing the cell substrate from chicken to human fibroblast HDCs did not affect the reactogenicity and immunogenicity of the new vaccine (AIK-C/MRC-5). The control viruses for this study was the Edmonston-Zagreb, adopted in human diploid cell (HDC) strain. In production and quality control of the measles vaccine, the safety, efficacy, neurovirulence, and genetic stability of the five measles vaccines were tested, based on WHO requirements (18). The neurovirulence in baby hamsters for all viruses adapted to HDC were not significantly changed, and all attenuated and virulent viruses, both before and after adaptation to HDC/MRC-5, were virulent in the baby hamsters except the Sugiyama virus.
The neurovirulence of the Sugiyama virus was increased for the day-old baby hamsters after 5 passages in HDC, although they were not virulent before adaptation. The high neurovirulence of the Sugiyama strain may have been due to either the new host cell membrane coating the virus or an increase in the multiplication of the more virulent particles (12). The plaque size increased after 20 passages in the MRC-5 cells for all viruses except the AIK-C strain. This indicated that the AIK-C measles vaccine strain was stable and homogenous. The vaccines were evaluated in a field trial using seronegative children in Iran (18, 19). The lowest clinical reactions to the AIK-C vaccine were compared to those of 5 other measles vaccine strains. The seroconversion was high for all vaccines, ranging between 95.5 and 100%, and the hemagglutination inhibiting (HI) antibody titre in the recipients’ sera ranged from 25.2 to 26.5 (Figure 2) (19). The comparative field trial results for the five vaccines produced in the human diploid cells, MRC-5, showed that those produced on the HDC substrate had a higher capacity for growth and replication in the human body and showed more immunogenicity than those produced on the other cell substrates (Figure 2) (20).
These findings were confirmed by further investigation and epidemiological studies, which indicated that administering AIK-C may be more effective than the other measles vaccine strains. Tidjani surveyed the serological effects of the Edmonston-Zagreb, Schwarz, and AIK-C measles vaccine strains in children at ages 4 - 5 months or 8 - 10 months (21). The detailed results of this serepidemiological study showed that high-dose Edmonston-Zagreb strains at 4 - 5 months of age is at least as effective as vaccinations with the standard strains at 8 - 10 months. The 55% of cases who were negative before vaccination at age 4 - 5 months, seroconversion rates were 96% with the AIK-C, 94% with the Edmonston-Zagreb, and 50% with the high-dose Schwarz vaccines. The children who were seropositive before the vaccination were estimated to be approximately 50% with the high-dose Edmonston-Zagreb and AIK-C strains and 10% with the Schwarz strain. Seroconversion rates for the vaccination at 8-10 months were 87% for the AIK-C and 68% for the Schwarz strains. Thus, vaccination with the high-dose Edmonston-Zagreb or the AIK-C strain at 4-5 months was as effective as the vaccination with the AIK-C strain at 8 - 10 months and more effective than the vaccination with the standard-dose Schwarz strain at 8 - 10 months (20). Immunizing 6 and 9 month old infants with AIK-C, Edmonston-Zagreb, Leningrad-16 and Schwarz strains of the measles vaccine also revealed standard-titres. AIK-C was more effective than the other measles vaccine strains for early immunization, efficacy, long-term immunogenicity, and long-term safety (21, 22).
The effects of the dosage and strain of live attenuated measles vaccines on serological responses in young infants was surveyed by Cutts et al. (23). They found that among children under 9 months of age, Edmonston Zagreb and AIK-C vaccines produced higher seroresponse rates than the Schwarz vaccine of an equivalent titre. Based on previous research, the AIK-C/MRC-5 was declared to be as potent and more immunogenic for controlling the measles epidemics which were common among Iranian children in 1950. The study of new vaccine viral strains began in 1963 and ended in late 1966. Production of pathogen-free, local calf serum was cost-effective and safe and helped to stabilize a working cell bank of HDCs for future vaccine production (24, 25).
3.2.3. Maternal Antibody, Age at Vaccination, and Immune Response
The effectiveness of the measles vaccine is impacted by the level of maternal antibodies to the measles virus during infancy and at the age of vaccination. The presence of maternally derived transplacental antibodies is important in evaluating the immunogenicity of the live measles vaccine in early infants because maternal antibodies interfere with the live measles vaccine virus. In Iran between 1967 and 1983, 1 dose of the monovalent AIK-C/RAZI vaccine was recommended for all children; however, the measles vaccination was not routinely offered in the public sector. To determine the maternal antibody level, the best age for vaccination, and the duration of passive immunity, the measles serum antibody (IgG) was measured by an HI assay in the cord blood at the time of birth (26, 27). The data showed that the maternal measles antibody protects the infant at a young age and then decreases as the child grow, suggesting that the measles vaccine should be given when the maternal immunity disappears. This study recommended 6 - 9 months as the optimal age to administer the measles vaccine. The second dose, given 3 - 4 months later, showed an increased humeral response after administration (26). These results were presented at the Congress of Vaccinations in tropical countries under WHO supervision and was accepted by Africa in 1977 (Guadeloupe vaccination in the tropics). Based on these data, vaccinating before 1 year could lead to a high risk of primary vaccine failure and subsequent infection in recipients. After the Islamic revolution in 1979, the recommended vaccination age was 12 months, based on the Ministry of Health & Education Program for Immunizations. Seroepidemiological surveys showed that 90 - 95% of the population at this age seroconverted after vaccination, compared with 55% in those aged less than 9 months. At present, the measles vaccination in Iran contains 2 doses of measles (MMR).
3.3. Measles Vaccine Efficacy
Vaccination age, passively acquired measles antibodies, immunological immaturity at vaccination, immunosuppressive conditions such as infection with human immunodeficiency virus type 1 (HIV-1) or, and in some circumstances, concurrent acute infections have critical roles in efficacy of measles vaccine (3). Frequently cited figures are that 90% of children develop protective antibody levels when given 1 dose of MV at 9 months of age, whereas 90 - 95% respond when vaccinated at 12 months (2, 3).
Surveys related to AIK-C measles-containing vaccines showed that this vaccine, either monovalent or combined with Rubella and Mumps, is generally recognized as safe and effective (1). Many serologic evaluations have demonstrated that over 50 years of using attenuated RAZI AIK-C/HDC MCVs elicits immune responses in most susceptible vaccine recipients particularly when handled and administered under ideal conditions. Epidemiological results of the measles vaccination in Iran changed the serepidemiological picture of this disease. If a measles reinfection occurs in a population with an HI antibody titre ≤ 1:16 (4 log2), it acts as a booster for increasing the antibody titre (28). Shafyi et al. reported that mass vaccination and EPI programs led to a natural circulation of the virus; therefore, reduced and natural boosting did not occur in the population. This phenomenon significantly reduced maternal antibody levels between 1990 and 1991 (28).
The necessity of measles immunizations in women before marriage was suggested by Shafyi A at the CVI conference in Geneva and was accepted for developing countries in Africa and Asia in 1992. In 2004, MR (Measles and Rubella) mass vaccinations of all Iranian women under 25 years of age helped to resolve this problem. Currently, Iran is trying to establish a target date for measles elimination. Although cases that have been imported from neighbouring countries affected by security conflicts in recent years pose a major challenge for eliminating measles in Iran.
3.4. Comparative Epidemiology of Measles; Pre- and Post-Vaccine Era in Iran
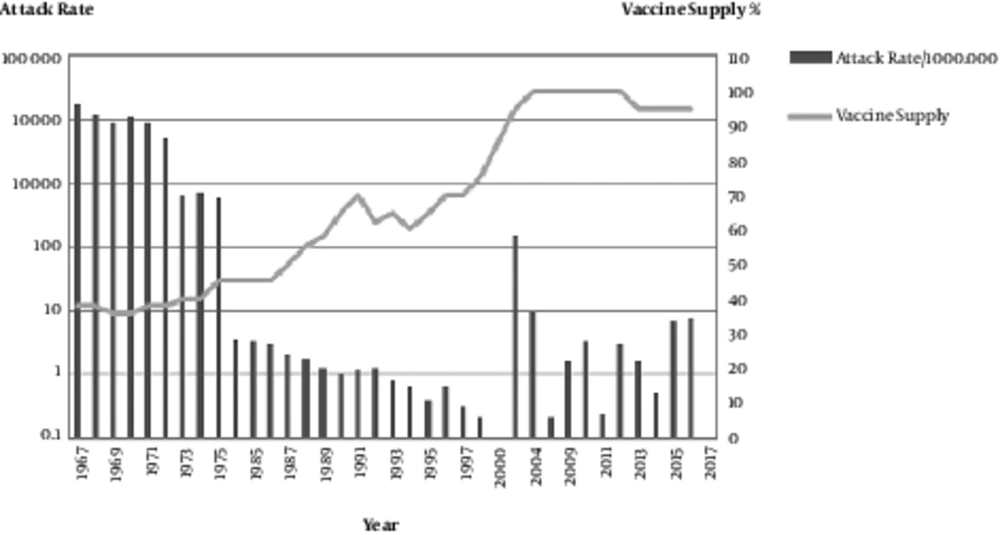
From the time of the vaccine licensure in 1963 until the end of 2016, over 88 million doses of the measles vaccine were produced at the Razi institute in Iran, and an estimated 95% of the 1- to 25-year-old population was vaccinated. Widespread vaccine production and administration during this period was followed by a 99% decrease in reported measles cases, resulting in large economic savings (Figures 1 and 2). In the pre-vaccine era, measles was a common infectious disease with high incidence, morbidity and mortality (29).
In the USA in 1958, with a population of 200 million, the number of measles-related deaths was 552, while in India there were 85,400 measles-related deaths in a population of 400 million. This means that for 1 death in the USA, there were 17 measles-related deaths in India. At the same time in Africa, Asia and Latin America there were 50 deaths per 100,000 residents (29). Immunization against measles in Iran started in 1967 with a population of 30 million. The annual incidence of measles over the period of 1967 - 1976 was 150,000 - 500,000, and the mortality was 5 - 10% with a higher rate of ≥ 10% in urban areas. In the early 1980s, there were 30,000 - 50,000 cases of measles annually. Between 1967 and 1983 there was no clear and efficient strategy for measles vaccinations. In 1979, after the Islamic revolution, the primary health system (PHC) with 95% accessibility in rural areas was established. Since 1984 systematic vaccinations against measles, which were initially monovalent and then became trivalent with MMR, for all children was initiated in Iran. The identified measles cases decreased significantly from 11,604 in 2002 to 724 in 2004 and to 1.6 cases per 1,000,000 persons in 2009 (30) (Figure 3).
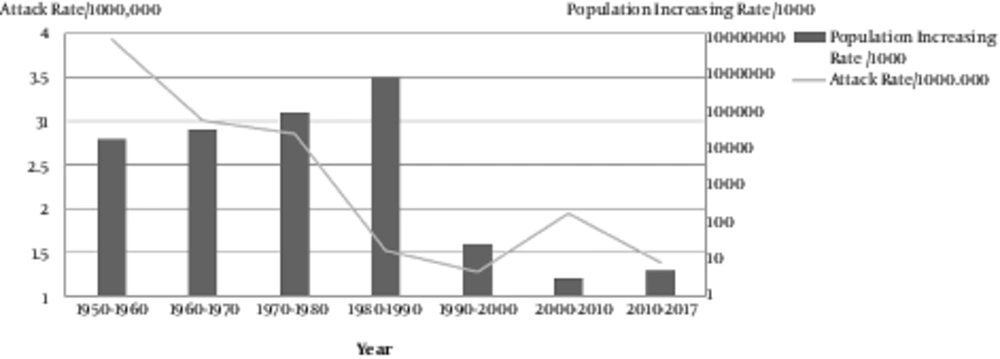
The successful and high-quality local measles vaccine resulted in < 1 case per million residents during 2004 - 2009. From 2009 until now, the number of measles cases that were laboratory-confirmed but not epidemiologically linked (positive and equivocal), as reported by the National Reference Laboratory, were approximately 1456 cases. The total population has doubled over the past 4 decades to an estimated 78 million in 2017. Recent surveys have revealed a greater than 92% seropositivity in Iranian children (31, 32) (Figure 4).
4. Conclusions
During the last 50 years, vaccine production, implementation and the widespread use of local EPI vaccines have all contributed to the decreased incidence of numerous preventable diseases and associated mortality in Iran. The development process of viral vaccine is a complex combination of knowledge and technology side along experience. Its development need to follow a fixed and standard pattern that fits the process well in general. We have described this complex process for Local measles vaccine in some standard details in this review, contains a schematic explaining the usual set of steps along the way from Isolation of virus to new local measles vaccine approval. Measles was introduced in population with a very high incidence and morbidity. This local vaccine production besides high coverage has led to a significant decrease in disease cases In Iran (Figure 3) (30, 32). A comparison between the period prior to the Local Vaccine production and implementation of national vaccination in Iran and 2017 showed a greater than 99% decline in the number of, measles (99.9%) cases. However, the result of seroepidemiology survey in some urban area reported that more effective national program needed to be organised for control and elimination of measles (33, 34). This production also had a major effect on economic and societal welfare. Eliminating the physical and disability defects caused by disease, making the healthy body for longer working years and higher productivity besides good educational results, and reducing healthcare costs.
Reductions in morbidity and mortality of measles disease have been so great that eradication has been proposed and believed to be feasible soon. Razi, as a member of the immunization family, hopes it will happen in Iran’s near future.
| Country of Origin Wild Isolate | Vaccine Strain | Cell Substrate | Reference |
|---|---|---|---|
| United State of America (Edmonston origin) | Edmonston B (1963) USA | Chicken Embryo Fibroblast | (35) |
| Edmonston-Zagreb | WI-38 (Human Lung Diploid) | (36) | |
| Beckenham & further attenuated Beckenham 31 | Chicken Embryo Fibroblast | (3) | |
| Schwarz (1965) USA | Chicken Embryo Fibroblast | (37) | |
| Moraten (1968) USA | Chicken Embryo Fibroblast | (38) | |
| AIK-C JAPAN | Chicken Embryo Fibroblast | (14) | |
| AIK/MRC5 IRAN | Human Lung Diploid cell (MRC-5) | (39) | |
| Russia Federation | Leningrad 16 (L16), USSR-58 | quail embryo cells (QEC), Chicken Embryo Fibroblast | (40, 41) |
| Yugoslavia | Milovanovic’s strains | Chicken Embryo Fibroblast | (41) |
| People’s Republic of China | Shanghai 191 | Chicken Embryo Fibroblast | (42) |
| JAPAN | CAM 70 | chorioallantoic membrane of chick embryo | (43, 44) |
| Sugiyama attenuated. Sugiyama178 (More attenuated) | Bovine Kidney | (9) | |
| Denken-Toyoshima, Biken-Sugiyama | chick-embryo amniotic membrane. Bovine Kidney | (45) |
![Correlation of measles HI antibody titre of Serum and Cerebrospinal Fluid (CSF) [R Value (Serum / CSF)] in SSPE patients from one hospital during 1975 - 1984 in Iran Correlation of measles HI antibody titre of Serum and Cerebrospinal Fluid (CSF) [R Value (Serum / CSF)] in SSPE patients from one hospital during 1975 - 1984 in Iran](https://services.brieflands.com/cdn/serve/3170b/40c0190f47697d49569cb63b4a96b0a92f3d8ce5/jjm-11-5-60725-i001-preview-preview.webp)