1. Background
Neurosurgery has mainly been used to treat cerebral hemorrhage diseases, brain tumors, and traumatic brain injury. Patients mostly have critical illness, various degrees of disturbance of consciousness, long surgical time, high surgical difficulty, etc., thus they are prone to infection. Intracranial infection after neurosurgery is amongst serious complications, with incidence rates of 1.52% to 6.6% (1, 2). It is mainly manifested as meningitis, cerebritis, brain abscess, etc. (3). The pathogens for intracranial infection may be bacteria, viruses, parasites, mycoplasma, chlamydia, mold, and rickettsia (4). Mostly caused by trauma, surgery, blood-borne abscesses, parasitic diseases, granulomas and tuberculosis (5), intracranial infection commonly occurs within 3 to 7 days after surgery (6).
By evaluating the effects of intraventricularly administered vancomycin, Chen et al. reported that intracranial infections after craniotomy were mostly induced by methicillin-resistant staphylococci (7). Recently, Fang et al. reported an intraventricular tigecycline therapy for intracranial infection with extremely drug resistant Acinetobacter baumannii, which was rather problematic because of multidrug resistance and difficult penetration through the blood-brain barrier (8).
The drug resistance of pathogenic bacteria, especially Staphylococcus aureus, is increasing annually, also hindering effective clinical treatment (9). In the presence of the blood-brain barrier, high-dose anti-infective drugs are required to treat intracranial infection for a long time. As a result, anti-infective treatment has become troublesome owing to aggravated resistance to antibacterial agents (10).
2. Objectives
Thereby, motivated to explore the distribution of pathogenic bacteria upon intracranial infection after neurosurgery and considering changes of resistance to antibacterial agents, the cerebrospinal fluid (CSF) bacterial cultures from infected patients from January 2013 to December 2015 were retrospectively analyzed. Based on the results, similar cases from January 2015 to June 2016 were effectively treated by rationally selecting antibacterial agents.
3. Methods
3.1. Ethics Statement
This study was approved by the ethics committee of the study hospital (code number: JCCH-20130105), and written consent was obtained from all patients.
| Type | 2013 | 2014 | 2015 |
|---|---|---|---|
| Total | 144 (68.9) | 270 (75.0) | 215 (77.9) |
| Escherichia coli | 9 (4.3) | 23 (6.4) | 28 (10.1) |
| Pseudomonas aeruginosa | 22 (10.5) | 45 (12.5) | 22 (9.8) |
| Klebsiellapneumoniae | 19 (9.1) | 36 (10.0) | 32 (11.6) |
| Acinetobacter baumannii | 42 (20.1) | 98 (27.2) | 69 (25.0) |
| Acinetobacter baumannii | 16 (7.7) | 56 (20.7) | 11 (4.0) |
| Other Gram-negative bacteria | 21 (10.0) | 30 (8.3) | 18 (6.5) |
Gram-Negative Bacteria in Cerebrospinal Fluid Cultures from 2013 to 2015 (n (%))
| Type | 2013 | 2014 | 2015 |
|---|---|---|---|
| Total | 65 (31.1) | 90 (25.0) | 61 (22.1) |
| Streptococci | 1 (0.5) | 8 (2.2) | 3 (1.1) |
| Staphylococcus aureus | 5 (2.4) | 4 (1.1) | 4 (1.4) |
| Staphylococcus hominis | 7 (3.3) | 18 (5.0) | 14 (5.1) |
| Enterococci | 11 (5.3) | 15 (4.2) | 6 (2.2) |
| Staphylococcus epidermidis | 20 (9.6) | 22 (6.1) | 13 (4.7) |
| Other staphylococci | 21 (10.0) | 23 (6.4) | 21 (7.6) |
| Staphylococcus auricularis | 15 (7.2) | - | - |
| Staphylococcus haemolyticus | - | 21 (5.8) | 20 (7.2) |
| Staphylococcus capitis | - | 29 (8.1) | 15 (5.4) |
Gram-Positive Bacteria in Cerebrospinal Fluid Cultures from 2013 to 2015 (n (%))
| Year | 2013 | 2014 | 2015 |
|---|---|---|---|
| Pathogenic bacteria (number of strains) | Acinetobacter baumannii (42) | Acinetobacter baumannii (98) | Acinetobacter baumannii (69) |
| Pseudomonas aeruginosa (22) | Acinetobacter lwoffii (56) | Klebsiella pneumoniae (32) | |
| Staphylococcus epidermidis (20) | Pseudomonas aeruginosa (45) | Escherichia coli (28) | |
| Klebsiella pneumoniae (19) | Klebsiella pneumoniae (36) | Pseudomonas aeruginosa (22) | |
| Acinetobacter lwoffii (16) | Staphylococcus capitis (29) | Staphylococcus haemolyticus (20) | |
| Staphylococcus auricularis (15) | Escherichia coli (23) | Staphylococcus capitis (15) | |
| Enterococci (11) | Staphylococcus epidermidis (22) | Staphylococcus hominis (14) | |
| Escherichia coli (9) | Staphylococcus haemolyticus (21) | Staphylococcus epidermidis (13) | |
| Staphylococcus hominis (7) | Staphylococcus hominis (18) | Acinetobacter lwoffii (11) | |
| Enterococci (5) | Enterococci (15) | Enterococci (6) |
Pathogenic Bacteria (Number of Strains) Ranking Top Ten from 2013 to 2015
3.2. Clinical Data for Bacterial Classification
Bacteria were collected from CSF cultures of all patients receiving neurosurgery at the department of neurosurgery, Jinhua and Wenzhou Hospital, between January 2013 and December 2015, and the same kinds of strains cultured from each case during the same infection were combined. The CSF samples were mainly collected from patients diagnosed with intracranial tumors, cerebral hemorrhage, cerebral vascular malformations, subarachnoid hemorrhage, and intra-spinal tumors.
3.3. Clinical Data for Selection of Antibacterial Agents
A total of 120 patients, who received neurosurgery at the hospital of this study, between January 2015 and June 2016, were selected, including 63 males and 57 females aged between 10 and 75 years old (49.32 ± 4.21). There were 26 cases of cerebrovascular disease, 17 cases of traumatic brain injury, 38 cases of intracranial tumor, 22 cases of spinal cord lesion, and 17 cases of hydrocephalus. All patients had fever symptoms, with body temperatures of 38°C to 40.5°C. After having intracranial infection, their consciousness was gradually weakened, and 9 patients had severe disturbance of consciousness, accompanied by symptoms, such as vomiting and headache.
Inclusion criterion was having intracranial infection (including meningitis, ventriculitis, brain abscess, as well as subdural and epidural abscess) after surgery. Exclusion criteria were as follows: 1, diagnosis of brain abscess and other infectious diseases before surgery; 2, diagnosis of brain abscess or inflammation after surgery; 3, death or discharged for various reasons within 2 days after surgery.
3.4. Diagnostic Criteria for Intracranial Infection
Patients were diagnosed with intracranial infection if they did not have fever, aggravated headache, vomiting, disturbance of consciousness or neck stiffness for other reasons after surgery, and diagnosis was confirmed according to the diagnostic criteria for hospital-acquired meningitis in the United States (11). At present, the diagnostic criteria for intracranial bacterial infection were as follows: symptoms and signs of high fever, headache, vomiting, and neck stiffness in clinical practice. The results of CSF bacterial culture and Gram staining are the gold standards for diagnosing intracranial infection, including white blood cell count of 1000 to 10000/μL, glucose level < 40 mg/dL (2.5 mmol/L), protein level > 50 mg/dL, and lactate level > 3.5 mmol/L in CSF.
3.5. Distribution Characteristics of CSF Bacteria and Selection of Antibacterial Agents
The hospital information system (HIS) was used to search and analyze the data of patients, who received neurosurgery at the hospital of the current research, from January 2015 to June 2016, and had positive CSF bacterial culture results after surgery, mainly including name, age, diagnosis, pathogenic bacteria, susceptible drugs, and resistant and intermediate drugs.
3.6. Sample Collection
The 120 patients underwent lumbar puncture to take CSF for routine, biochemical, and bacterial culture examinations on the day when intracranial infection was found.
3.7. Treatment of CSF Samples
Turbid or purulent CSF was directly smeared on a glass slide, stained and examined by microscopy. Colorless transparent CSF was centrifuged to collect the precipitate that was then smeared on a glass slide and subjected to Gram staining. The infected bacteria were preliminarily classified according to staining results and morphological characteristics.
3.8. API Bacterial Identification
A single bacterial colony was isolated and prepared into a suspension. Next, 5 mL of sterile water was added in to a culture box, into which strips were placed. Meanwhile, the bacterial suspension was inoculated in small reaction tubes. Afterwards, designated wells were covered by paraffin oil, and the culture box was closed. The strips were thereafter placed in a 37°C incubator for 18 hours. According to the kit’s instructions (bioMerieux, France), reagents were added to appropriate wells. The results were recorded and analyzed by the Apilab software (France).
3.9. Bacterial Analysis and Identification
Pathogenic bacteria isolated from the CSF samples mentioned above were classified using the VITEK-60 analyzer and corresponding identification cards. The identified pathogenic bacterial strains were subjected to susceptibility tests with commonly used antibacterial agents in clinical practice, using the K-B method. The susceptibility cards were all purchased from Bio-Merieux (France). The test results were determined referring to the national committee for clinical laboratory standards (USA). The quality control strains were Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), and S. aureus (ATCC 25923). All the above strains were provided by the national center for medical culture collections (China).
3.10. Treatment Methods
According to the results of bacterial culture, the patients were treated by proper antibiotics with the following methods: 1, Before the bacterial culture results were obtained, the patients were treated with blood-brain barrier-penetrating drugs, such as chloramphenicol and panipenem; 2, after the bacterial culture results were acquired, appropriate antibiotics were selected; 3, appropriate methods of administration were selected according to the state of patients. The patients with elevated CSF cell counts were intravenously injected with antibiotics. The patients with barely increased intracranial pressures were treated by intravenous and intrathecal injections. The patients with severe bacterial infection were treated by intravenous and intrathecal injections. Meantime, lumbar catheterization was employed for drainage; 4, vancomycin or ceftriaxone sodium was selected when the patients were treated by intrathecal administration; 5, during treatment, attention was paid to maintaining the electrolyte and pH balance.
3.11. Criteria for Therapeutic Effects
Cured: 1, Body temperature was within the normal range for 3 consecutive days; 2, the results of CSF bacterial culture were negative; 3, the white blood cell count was less than 8 × 106/L; 4) there was no meningeal irritation sign.
4. Results
4.1. Distribution Characteristics of Bacteria in CSF
A total of 845 pathogenic bacterial strains (209 in 2013, 360 in 2014 and 276 in 2015) were isolated from CSF samples and cultured, of which 629 were Gram-negative bacteria, accounting for 74.4%. The Gram-negative bacteria were mainly Acinetorbacter baumannii, Klebsiella pneumoniae, P. aeruginosa, A. lwoffii, and Escherichia coli. Particularly, the A. baumannii strains were ranked first each year (Table 1). On the other hand, there were 216 Gram-positive bacteria, which accounted for 25.6% and were mainly S. epidermidis, S. hominis, and S. aureus (Table 2). Notably, S. epidermidis was the most common from 2013 to 2015. The types of pathogenic bacteria ranked as the top 10 did not change during 3 years (Table 3).
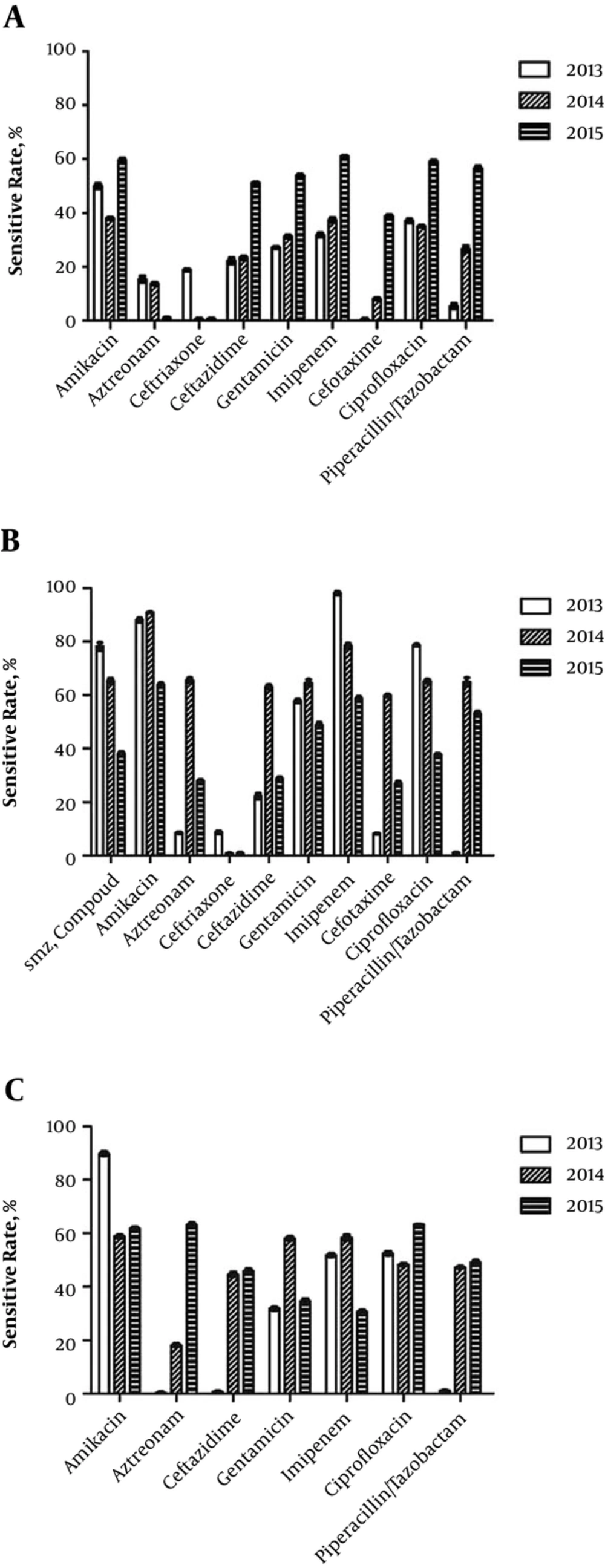
4.2. Susceptibility of Gram-Negative Bacteria in CSF to Common Antibacterial Agents
In 2013 and 2014, the susceptibility rates of A. baumannii toward all tested drugs were lower than 40%. From 2013 to 2015, 52 A. baumannii strains were detected, of which 23 were pan-resistant, being only susceptible to polymyxin. Only the susceptibility rates towards aztreonam and ceftriaxone sodium were lower than 20% (Figure 1A). The number of K. pneumoniae strains increased annually, and their susceptibility rates to ceftazidime and imipenem significantly decreased in 2015 compared with those in 2014 (Figure 1B). Pseudomonas aeruginosa strains had high susceptibility rates to all drugs, with the rates toward gentamicin and imipenem being < 40% in 2015 and < 20% in 2014 (Figure 1C).
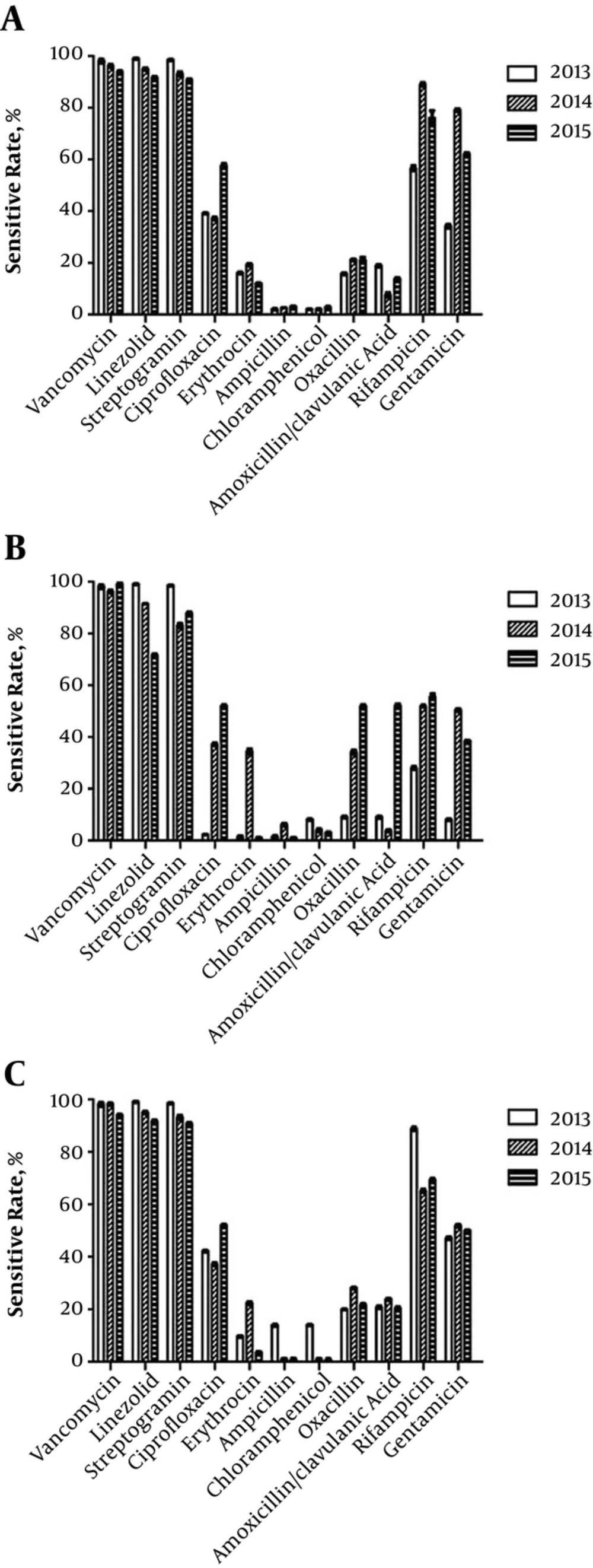
4.3. Susceptibility of Gram-Positive Bacteria in CSF to Common Antibacterial Agents
Gram positive bacteria were mostly methicillin-resistant staphylococci. For example, > 90% of S. epidermidis (Figure 2A), S. aureus (Figure 2B), and S. hominis (Figure 2C) strains produced β-lactamase. Therefore, they were resistant to β-lactams, such as ampicillin, penicillin, oxacillin, and amoxicillin/clavulanic acid. They were only sensitive to vancomycin, linezolid, streptogramin, and rifampicin.
4.4. Selection of Antibacterial Agents
Based on the above results, HIS was used to search and analyze the data of 120 patients, who received neurosurgery at the hospital of the current research from January 2015 to June 2016, and had positive CSF bacterial culture results after surgery. The distribution of pathogenic bacteria was consistent with that mentioned above. After treatment based on the susceptibility rates of pathogenic baccompared with those in 2014teria to the tested antibacterial agents, 101 patients were cured by vancomycin and linezolid (cure rate: 84.17%).
5. Discussion
At present, there is a increasing threat worldwide by drug-resistant bacteria. A report of the national nosocomial infections surveillance in 2004 showed that at intensive care units (ICU), patients with methicillin-resistant Staphylococcus aureus (MRSA) accounted for 63.3% of all cases infected by S. aureus (12). Currently, the incidence rates of intracranial infection after neurosurgery range from 1% to 10% (13). Especially, the patients receiving second surgery due to long-term ventricular drainage for subcutaneous effusion, after surgery for cerebrospinal fluid leaks and postoperative emergencies, are more prone to intracranial infection (14). The isolation rate of MRSA was, as revealed by a Chinese report, 69.5% among all S. aureus infections. The mortality rate of MRSA was 20 folds that of methicillin-sensitive S. aureus (15). Therefore, it is crucial to select proper antibacterial agents at first in the treatment of MRSA infections. As a glycopeptide, vancomycin is always given priority in treating MRSA, yet its minimal inhibitory concentration has been increasing annually due to elevated dosage and frequency as well as appearance of vancomycin-resistant S. aureus and enterococci, thus the treatment outcomes of intracranial infection remains unsatisfactory.
Although over 3000 cases of CSF samples were subjected to bacterial culture and examination each year in the hospital of the current research, the proportion of positive cultures was < 10%, probably because long-term prophylactic use of antibacterial agents inhibited bacterial growth and gave inaccurate results. Thus, intracranial infection has often been treated empirically. To solve this problem, this research herein isolated 845 pathogenic bacterial strains from CSF samples for retrospective analysis, of which 629 were Gram-negative bacteria, mainly being A. baumannii, K. pneumoniae, P. aeruginosa, A. lwoffii and E. coli. The isolation rate of bacteria from the genus Acinetobacter (mainly A. baumannii) ranked first among those of all Gram-negative bacteria, which may be associated with subsequent ICU treatment for critical cases after surgery. Besides, A. baumannii had severe multidrug resistance, which was only susceptible to polymyxin in 2015 with a rate of 87%.
Moreover, the susceptibility rates of A. baumannii towards each tested drug increased annually, which could mainly be ascribed to the following reasons. First, imipenem was seldom prescribed by neurosurgeons because of adverse reactions, such as epilepsy. Second, amikacin, gentamicin, and ciprofloxacin were not used at the hospital from 2013 to 2015. Third, patients had low tolerance to piperacillin and tazobactam, thus they were barely used. Recently, outbreaks of infections induced by pan-resistant A. baumannii, including ventilator-associated pneumonia, skin and soft tissue infections, urinary tract infection, secondary meningitis and bloodstream infection, have been witnessed worldwide (16). In contrast, related intracranial infection has seldom been reported. Nevertheless, once this infection occurs, only polymyxin and sulfonamide injections, which cannot be easily purchased, are suitable for treatment. Thus, it is necessary to establish an effective bacterial drug resistance monitory system based on the cooperation between the microorganism, nosocomial infection, and clinical departments in hospitals (17).
In this study, the number of K. pneumoniae strains increased from 9 to 14 in during 3 years. Though it was susceptible to imipenem and amikacin, the susceptibility rates decreased annually. Furthermore, since cephalosporins (e.g. cefuroxime and ceftriaxone) have been widely used for the prophylaxis of neurosurgery, Gram-negative bacteria have become more resistant to 3rd generation cephalosporins and new β-lactams (18). Of all cephalosporins, cefepime had a high susceptibility rate. In 2013 and 2015, the susceptibility rates of P. aeruginosa was 69% and 70% respectively, yet that of Acinetobacter bacteria, K. pneumoniae, and E. coli was lower than 50% in 2015.
On the other hand, 216 Gram-positive bacteria (25.6%) were cultured from 2013 to 2015, which were mainly β-lactamase-positive, and methicillin-resistant staphylococci. Only vancomycin, linezolid, and rifampicin worked for this group. However, the susceptibility rates of vancomycin and linezolid was reduced annually after being widely used. Hence, it is of great significance to effectively prevent further progression of vancomycin resistance in the treatment of postoperative intracranial infection. Staphylococci-induced meningitis can be well treated by linezolid and daptomycin (19, 20), and 70% of the former can penetrate the blood-brain barrier. Linezolid has not been approved by the US FDA to treat intracranial infection hitherto, yet it has definite therapeutic effects on that induced by vancomycin-resistant staphylococci and enterococci in clinical practice (21).
Finally, 120 eligible patients from 2015 to 2016 were treated based on retrospective results and HIS, instead of empirical use of antibacterial agents. After treatment based on the susceptibility rates of pathogenic bacteria to the tested antibacterial agents, 101 patients were cured by vancomycin and linezolid (cure rate: 84.17%), suggesting that the findings of retrospective analysis were of high clinical value.
6. Conclusions
In summary, this study retrospectively analyzed the distribution characteristics and drug resistance changes of pathogenic bacteria in CSF from patients with intracranial infection receiving neurosurgery, based on which antibacterial agents were rationally selected for treating subsequently enrolled cases. Since the treatment outcomes after rational selection of antibacterial agents (i.e. vancomycin and linezolid) were satisfactory, the findings of retrospective analysis are valuable, paving the way for further studies in clinical practice.