1. Background
Classic Klebsiella pneumoniae are multidrug-resistant strains associated with nosocomial infections outbreak and its hypervirulent strains are related to severe community-acquired infections (1, 2). During the past decade, CTX-M enzymes have become the most prevalent extended spectrum beta-lactamase (ESBL) enzymes in nosocomial infections caused by K. pneumoniae (3, 4). Klebsiella pneumoniae strains with plasmids harbouring blaCTX-M-15 have been detected in clinical isolates worldwide (5).
Bacteria can acquire antimicrobial resistance by the spread of transmissible plasmids, which may also carry virulent traits. The acquisition of antibiotic resistance and virulence determinants may represent a survival benefit to the bacteria (6). Several virulence genes such as the regulator of mucoid phenotype A gene (rmpA), iron acquisition system aerobactin (iucB) and lipopolysaccharide synthesis (wabG) have been detected and determined to be associated with the hypervirulent variant of K. pneumoniae. Both aerobactin system and regulator of mucoid phenotype A gene are found almost in all reported hypervirulent variants of K. pneumoniae strains located on a large plasmid. The important roles of rmpA and wabG genes are in capsular polysaccharide and lipopolysaccharide syntheses, respectively (7, 8).
Capsular polysaccharide (K antigen) is a major virulence factor responsible phagocytosis and serum killing resistance. At least 78 capsular serotypes have been identified while, serotype-related variation in infection severity have been observed. Strains with capsular serotypes K1 and, to a lesser extent, K2, K5, K20 and K54, have been recognized as the predominant virulent strains (8, 9).
2. Objectives
The aim of this study was to identify the mucoviscosity, iron acquisition, lipopolysaccharide synthesis and serotypes of K. pneumoniae strains isolated from clinical specimens and to evaluate the association among virulence traits and blaCTX-M-15 gene.
3. Methods
3.1. Ethics Statement
This study has been approved by the ethics committee of Kerman University of Medical Sciences (Code Number: IR.KMU.REC.1394.496), and written consent has been obtained from all patients.
3.2. Bacteria and DNA Extract
From October 2014 to May 2015, 103 non-duplicate non-consecutive K. pneumoniae were collected from patients referred to different clinical laboratories and hospitals in Kerman city (southeastern Iran). Clinical isolates were mostly from urine, wound, tracheal secretions and blood. The isolates were identified by standard biochemical and bacteriological methods. Isolates were then preserved at -70°C in brain-heart infusion (BHI) (HiMedia, India) broth with 30% glycerol until further tests. Bacterial genomic DNA was extracted from isolates using a kit (Topaz gene research, Iran) according to the manufacturer’s instructions.
3.3. Virulence Genes
Specific PCR primers for the detection of rmpA, wabG and iucB genes were previously described by Chen et al. (8).
3.4. Serotypes
K1, K2, K5 and K20 serotypes were identified by the detection of capsular polysaccharide (K serotype-specific) wzx and wzy alleles, using the multiplex PCR method as previously described (8).
3.5. Detection of blaCTX-M-15 Gene
All isolates were screened by PCR for the blaCTX-M-15 gene, as described by Messai et al. (10).
3.6. String Test
The presence of hypermucoviscosity was evaluated using the string test, and a positive result was defined as formation of a viscous capsular string > 5 mm in length by stretching bacterial colonies on an agar plate (11).
3.7. Statistical Analysis
Descriptive and inferential statics were used for data analyse. Differences in virulence genes and serotypes with antimicrobial resistance gene were compared using, SPSS version 19.0 (SPSS, Inc), with a P value of P < 0.05 indicating statistical significance.
4. Results
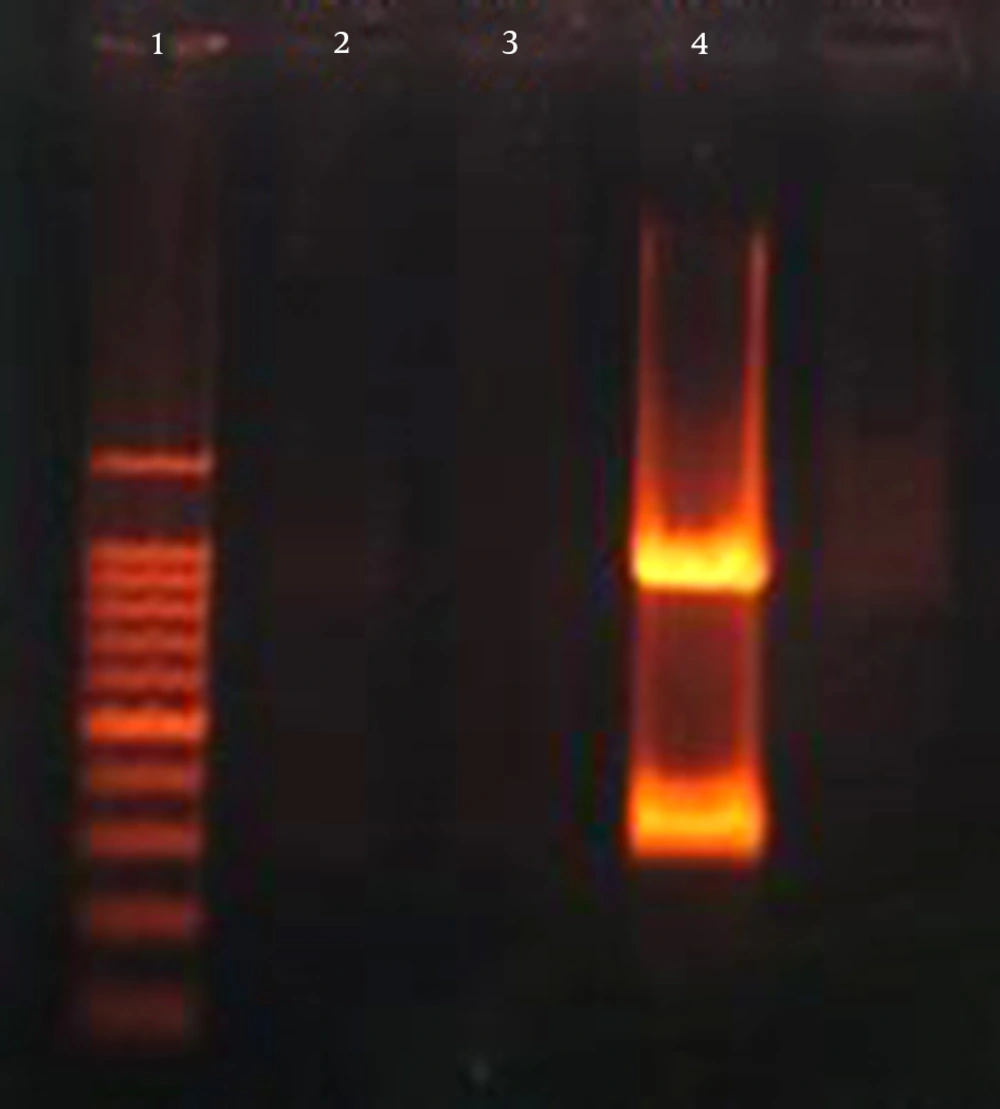
The K. pneumoniae isolates were screened for the presence of virulence genes. In total, 62 out of 103 (60.2%) K. pneumoniae isolates harbored at least one of the virulence genes chosen for analysis. The most prevalent virulence gene was wabG (59.2%; 61/103), followed by iucB (3.9%; 4/103). The regulator of mucoid phenotype encoding gene rmpA was present in 2.9% (3/103) of the isolates (Figures 1, 2) (Table 1).
| Variables | Gene | Total, No. (%) |
|---|---|---|
| Serotypes | K1 | 3 (2.9) |
| K2 | - | |
| K5 | - | |
| K20 | 4 (3.9) | |
| Virulence trait | wabG | 61 (59.2) |
| iucB | 4 (3.9) | |
| rmpA | 3 (2.9) | |
| ESBL | blaCTX-M-15 | 47 (45.6) |
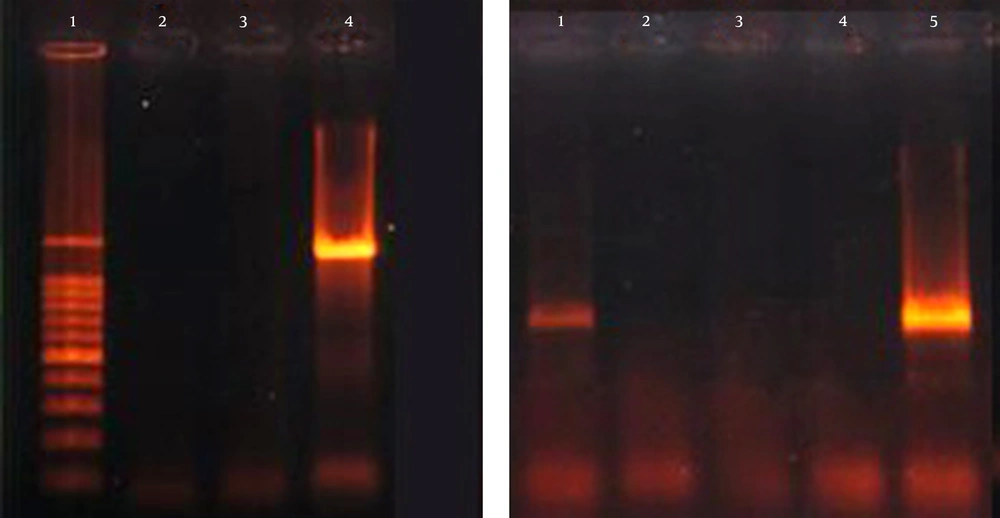
Overall, 7 of 103 (6.8%) K. pneumoniae isolates were positive for at least one of the examined serotype genes. The presence of K20 in 3.9% (4/103) of the isolates was the most prevalent represented. The K1 serotype was the least prevalent in three isolates (Figure 3). None of the K. pneumoniae isolates were positive for K2 and K5 serotypes. Among the isolates positive for K1 serotype, 0.9% (1/103) was in combination with wabG, iucB and rmpA genes (hypervirulent), 0.9% (1/103) of isolates in combination with blaCTX-M-15, wabG, iucB and rmpA genes and 0.9% (1/103) was accompanied by blaCTX-M-15, wabG and iucB genes. Also, among the isolates that possessed the K20 serotype, 2.9% (3/103) were in combination with wabG gene.
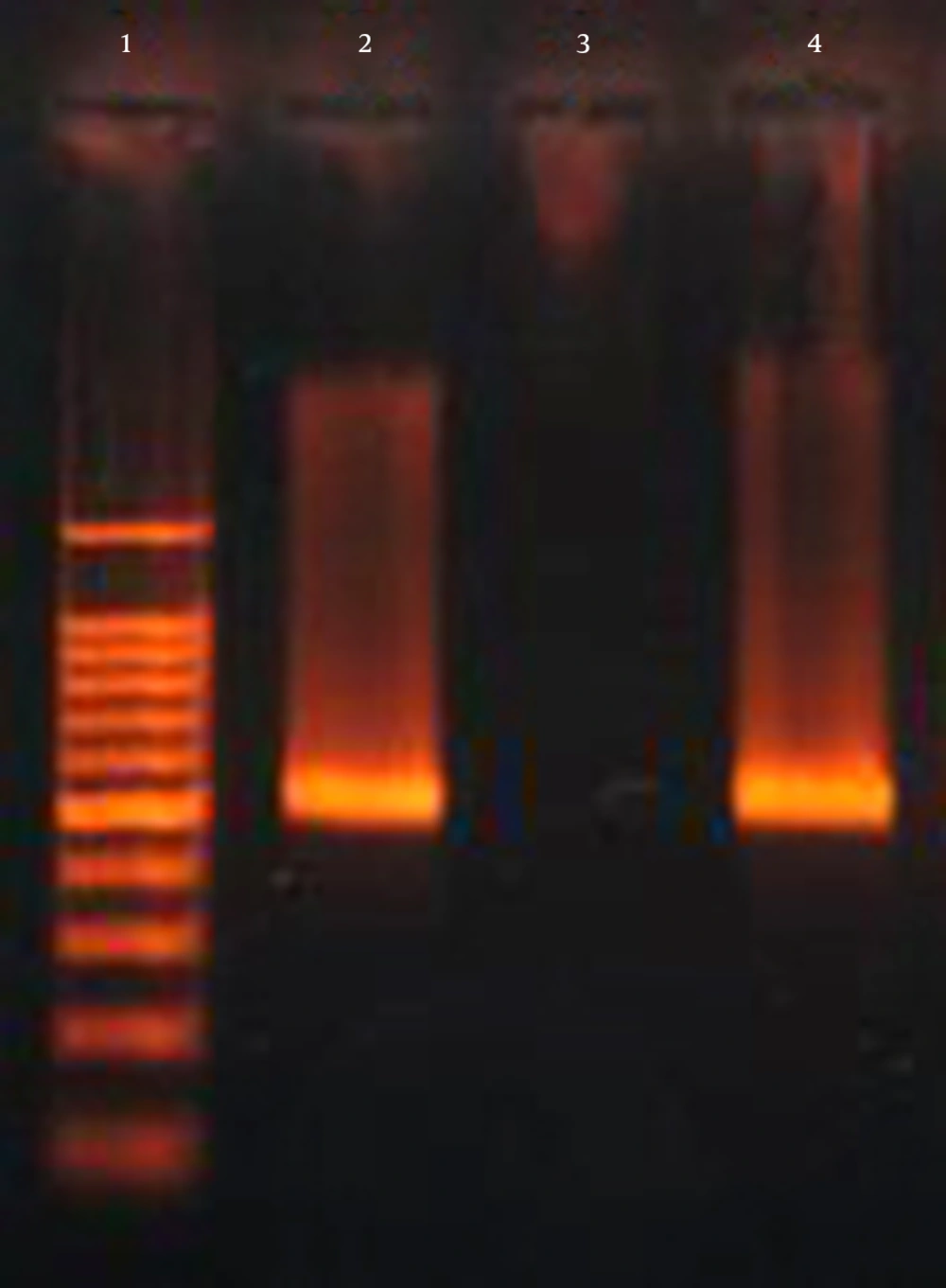
Based on the results of the string test, hypervirulent phenotypes were identified in 3 (2.9%) of the isolates, whit only one (0.9%) isolate having the characteristic hypervirulent phenotype, was positive for K1, rmpA, wabG and iucB genes. The ESBL encoding gene blaCTX-M-15 was observed in 45.6% (47/103) of isolates (Figure 4 and Table 1). Of the 47 K. pneumoniae isolates that possessed blaCTX-M-15 gene, 28 (59.6%) isolates were positive for wabG gene. Bivariate analysis confirmed that this difference between the number of isolates was significant (P = 0.000). Among 47 isolates that possessed blaCTX-M-15 gene, three isolates were positive for rmpA gene (P > 0.05). There was no significant difference in blaCTX-M-15 gene to iucB gene (3/47 isolates) (P > 0.05). In addition, there was no association between the presence of blaCTX-M-15 gene and the K1 (2/47 isolates) and K20 (2/47 isolates) serotypes (P > 0.05).
| Variables | Virulence Genes and Serotypes Pattern | No. |
|---|---|---|
| blaCTX-M-15a | wabG, rmpA, iucB, K1 | 1 |
| wabG, iucB, K1 | 1 | |
| wabG, rmpA, iucB | 1 | |
| wabG, K20 | 2 | |
| wabG | 23 | |
| - | 19 | |
| Total | 47 |
aIsolates possessed blaCTX-M-15 gene.
5. Discussion
Klebsiella pneumoniae is known as an important reservoir for various types of ESBL enzymes, and has spread worldwide (12). The high rate of CTX-M producing K. pneumoniae prevalence and its widespread dissemination in Iran is causing concern (13). Virulence genes thought to be associated with invasive community-acquired infections include lipopolysaccharide synthesis, iron acquisition system, hypermucoidy and specific polysaccharide capsule serotypes (2). The present study showed a correlation between the epidemiology of specific virulence genetic traits and serotypes with blaCTX-M-15 gene; a first step toward understanding whether there is a link between virulence and resistance. The results from previous researches have shown that there is a link between resistance and virulence in Escherichia coli isolates (6). Lavigne et al. demonstrated that 14 virulence factors were less prevalent in ESBL isolates than in susceptible E. coli isolates (14). In another study by Lee et al. showed that CTX-M producers of E. coli isolates had fewer virulence factors than TEM-producing isolates (15).
The results showed a high (45.6%) prevalence of blaCTX-M-15 gene among the K. pneumoniae isolates. The existence of lipopolysaccharide synthesis encoding gene (wabG gene) was one of the most common virulence genes identified among the studied isolates. The findings by Chen et al. showed that there was high rate of wabG gene among 327 K. pneumoniae clinical isolates in China (8). According to the results of the current study, K1 serotype was detected in ~3% of the isolates. Lipopolysaccharide and capsular polysaccharide components are important pathogenic traits in K. pneumonia-caused bacteremia and pneumonia (16). The K1 strains were wabG, iucB, rmpA and blaCTX-M-15-positive genes, which indicate that most of the K1 isolates tested in this study, were closely related to hyper-virulence and ESBL. Previous studies have shown that the K1 serotype K. pneumoniae isolated from liver abscesses is less resistant to antibiotics when compared with other serotypes (17, 18), whereas previously published data indicated that the K1 serotype is associated with antibiotic resistance (19, 20).
Of the virulence genes, wabG was significantly more common in strains producing the blaCTX-M-15 group ESBLs. Derakhshan et al. compared the occurrence of rmpA and wcaG genes with the production of class 1 integron (intl1) among K. pneumoniae strains isolated from clinical specimens. The prevalence of wcaG was more frequent in the intl1 producing K. pneumoniae (21). The findings of this study indicated that the numbers of wabG-carrying isolates were more common in strains producing the blaCTX-M-15 gene (P < 0.05). The results of the current study showed that the rmpA gene was detected in quite a low frequency compared to other isolates. The rmpA gene encodes a transcriptional activator of capsular polysaccharide which is one of the major contributing factors of virulence in K. pneumoniae, promoting biofilm formation and is closely associated with the hypermucoviscous phenotype (8, 22). Yu et al., showed that rmpA-positive isolates were related to the clinical syndrome caused by invasive strains and with the hypermucoviscous phenotype (23). Another study that demonstrated the relationship between rmpA, virulence and specific polysaccharide capsule serotypes is yet to be elucidated (9).
In the present study, the aerobactin encoding gene iucB was present in 3.9% of isolates. Several molecular epidemiologic studies were suggestive that hypervirulent K. pneumonia strains produced more aerobactin than classical K. pneumoniae strains and it may be that this factor enhanced the virulence of hypervirulent K. pneumoniae (24, 25). In this regard, Li et al. suggested that hypervirulent K. pneumoniae has the potential to acquire significant antibiotic resistance (26).
6. Conclusions
In summary, the data presented in this study suggest that blaCTX-M-15 producing K. pneumoniae contain high levels of wabG virulence gene. However, the association of virulence traits and antibiotic resistance in different studies are not clear. More in depth molecular epidemiologic researches on the genetics between virulence factors and antibiotic resistance are sorely needed, to fully understand the relationship between virulence and resistance genes in K. pneumoniae strains.