1. Background
Acinetobacter is a Gram-negative, polymorphic, nonmotile, strictly aerobic bacterium, which can be easily grown in a conventional laboratory environment (1). Different species of Acinetobacter cause urinary tract infections, wound infections, meningitis, endocarditis, peritonitis, and dermal and soft tissue infections (2). This opportunistic bacterium can survive on various surfaces, including patient’s skin and used medical equipment. Thus, prolonged hospitalization in the intensive care unit, severe disease, immune system deficiency, burn injuries, long-term use of antimicrobial agents, and catheter use are risk factors for Acinetobacter infection (3).
Acinetobacter baumannii is more prevalent than other strains of Acinetobacter, and it is the most common cause of bacterium-induced infections. To treat infections caused by Acinetobacter, various antibiotics, including β-lactams, fluoroquinolones, and aminoglycosides, are used; however, inappropriate and indiscriminate use of these antibiotics in recent years has led to the emergence of strains that are highly resistant to antimicrobial agents (4). Such resistance can be innate or caused by genetic resistance factors (5). Multidrug resistance (MDR) of Acinetobacter has been reported (6). The production of β-lactamase enzymes is a major mechanism of antimicrobial resistance and a major problem in the treatment of various infectious diseases. These enzymes can disable β-lactam antibiotics by hydrolysis of the central core of the antibiotics. Of various β-lactamases, ESBLs have greater functions (7). The vast majority of ESBLs are the result of mutations in CTX-M, SHV (sulphydryl variable), and TEM (Temoneira) genes (8).
With the increase in drug resistance, ESBLs limit the choice of common antibiotics that can be used. Drug resistance can be caused by the transfer of resistant genes carried on plasmids and simultaneous transfer of other antibiotic-resistance genes (9). In some cases, due to high resistance to common antibiotics, ESBL-producing bacteria can lead to mortality by disrupting the treatment of infectious diseases (10). Given the high prevalence of hospital infections, studies on the causative microorganisms, including their antibiotic resistance patterns, are of paramount importance.
2. Objectives
There are no recent comprehensive studies in Kermanshah on the prevalence of CTX-M, TEM, and SHV genes in A. baumannii. Thus, the aim of the present study was to determine the prevalence of extended-spectrum β-lactamases (ESBLs) and antibiotic resistance patterns in A. baumannii isolated from clinical samples at Imam Reza hospital in Kermanshah, Iran, using phenotypic and genotypic methods.
3. Methods
3.1. Ethics Statement
The ethics committee of Kermanshah University of Medical Sciences approved the study protocol (No.: 1394.250).
3.2. Bacterial Isolates and Identification
In this descriptive cross-sectional study, 385 clinical samples were collected from different sources (endotracheal tubes, blood, sputum, urine, catheters, wounds, and various liquids) at Imam Reza hospital in Kermanshah from January 2016 to November 2016. Following collection, the samples were transported to the laboratory, cultured on blood agar and Mac Conkey agar (Merck, Germany), and then incubated for 24 hours at 37°C. In total, 80 isolates of A. baumannii were identified and confirmed using standard biochemical tests, including growth on citrate media and nonfermentable lactose, oxidase and catalase activity, immobility on SIM medium, ALK/ALK patterns on TSI medium (Himedia CO, India), and lack of pigment production. The PCR method using OXA-51 gene was used for final confirmation of the Acinetobacter isolates (11).
3.3. Antibiotic Susceptibility Testing
The antibiotic resistance of the isolates was determined using Kirby–Bauer disk diffusion method, according to the guidelines of the clinical and laboratory standards institute (CLSI) (12). To this end, 14 antibiotic discs (MAST, U.K.) were used: gentamicin (10 µg), amikacin (30 µg), tobramycin (10 µg), imipenem (10 µg), meropenem (10 µg), ceftazidime (30 µg), cefotaxime (30 µg), ceftriaxone (30 µg), ciprofloxacin (5 µg), levofloxacin (5 µg), piperacillin/tazobactam (10/100 µg), ticarcillin/clavulanic acid (10/75 µg), ampicillin/sulbactam (20 µg), and polymyxine B (300 µg). The A. baumannii suspension was spread on Mueller-Hinton agar medium (Himedia CO, India) after incubation at 37°C and compared with a 0.5 McFarland standard using the lawn culture method. The disks were then placed on them. After incubation in an incubator for 24 hours, the growth inhibition diameter was measured and compared with that listed in CLSI tables. Escherichia coli ATCC 25922 was used for quality control of the reaction. Acinetobacter isolates showing resistance to three or more antibiotic strains were regarded as MDR strains.
3.4. Amplification of blaTEM, blaSHV, and blaCTX-M Genes by the PCR Method
To identify ESBL-producing isolates, the combined disk method was used. First, a standard concentration of A. baumannii was lawn cultured on Mueller-Hinton medium. Then, discs containing ceftazidime, ceftazidime/clavulanic acid, cefotaxime, and cefotaxime/clavulanic acid were placed 15 mm away from each other on the plates. After incubation for 24 hours at 37°C, the zone of inhibition around the disk containing clavulanic acid only was compared to that of the same ESBL disk as the enzyme inhibitor was measured. If the zone of inhibition around the disk containing clavulanic acid was greater than or equal to 5 mm as compared to that of the same disk without clavulanic acid, the strain was considered an ESBL-producing strain in accordance with CLSI guidelines. A standard Escherichia coli strain, ATCC 25922, was used as positive control. For PCR, DNA was extracted from the isolates using boiling method, and the frequencies of SHV, TEM, and CTX-M genes were investigated using specific primers (Takapou Zist Co., Iran), as shown in Table 1 (13, 14).
| Gene | Nucleotide Sequence (5’ - 3’) | Annealing, °C | Product Size, bp |
|---|---|---|---|
| blaSHV | 5’-CTTTACTCGCTTTATCG-3’ | 53 | 868 |
| 5’-TCCCGCAGATAAATCAC-3’ | |||
| blaTEM | 5’-GAGTATTCAACATTTCCGTGTC-3’ | 45 | 800 |
| 5’-TAATCAGTGAGGCACCTATCTC-3’ | |||
| blaCTX-M | 5’-ATGTGCAGTACCAGTAAGGT-3’ | 53 | 594 |
| 5’-TGGGTAAAGTAGGTCACCAGA -3’ |
3.5. PCR Assay
The PCR assay was conducted using 12.5 mL Master Mix (SinaClon Company, Iran), 1 µg of each primer, 3 µg bacterial DNA, and distilled water to obtain a final volume of 25 mL. Except for the annealing temperature, the PCR reaction temperature was the same for all the three β-lactamase genes. It consisted of initial denaturation for 4 minutes at 94°C, followed by 35 cycles of denaturation for 1 minute at 94°C, amplification at 72°C for 40 seconds, and final amplification at 72°C for 5 minutes (Table 1). Finally, the PCR products were examined using electrophoresis and staining with ethidium bromide.
3.6. Statistical Analysis
Chi-square and Fisher’s tests were conducted. All statistical analyses were performed using statistical package for the social sciences (SPSS), version 20.
4. Results
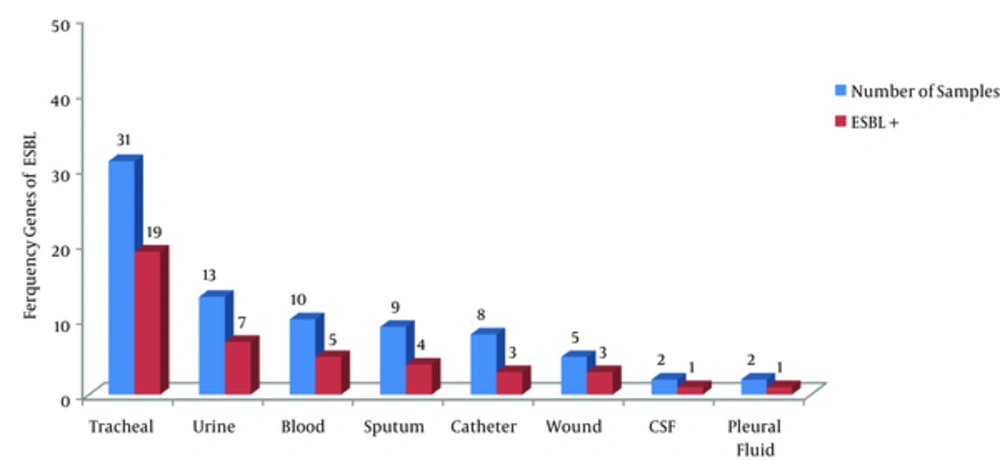
In the 385 clinical samples examined in this study, 80 (20.8%) isolates of A. baumannii were detected. In the study, 43 (53.8%) samples were obtained from males, and 37 (46.2%) from females, with a mean age of 48.38 ± 14.7, ranging from 19 to 82 years. Majority of the isolates were detected in respiratory samples (n = 40, 50%). The lowest detection frequency was in cerebrospinal fluid and pleural fluid (n = 4, 5%) (Figure 1).
As shown by the antibiotic resistance patterns of A. baumannii given in Table 2, the highest resistance was observed against ceftriaxone (100%) and amikacin (96.2%), whereas the lowest resistance was against polymyxin B (13.8%) and ampicillin/sulbactam (52.8%). Among the isolates, 50 (62.5%) showed MDR against the antibiotics tested. Based on the results of the confirmatory combined-disk test, 43 (53.8%) of the 80 A. baumannii isolates showed ESBL production. The frequency of ESBL genes in the clinical samples is shown in Figure 1. ESBL genes were not significantly correlated with the age and gender of the patients (P > 0.05).
| Antibiotic | A. Baumannii Isolated | ||
|---|---|---|---|
| Resistant | Intermediate | Susceptible | |
| Ceftazidime | 76 (95) | 0 | 4 (5) |
| Ceftriaxone | 80 (100) | 0 | 0 |
| Cefotaxime | 73 (91.2) | 1 (1.2) | 6 (7.5) |
| Ciprofloxacin | 64 (80) | 4 (5) | 12 (15) |
| Levofloxacin | 57 (71.2) | 10 (12.5) | 13 (16.2) |
| Gentamicin | 63 (78.8) | 2 (2.5) | 15 (18.8) |
| Amikacin | 77 (96.2) | 0 | 3 (3.8) |
| Tobramycin | 54 (67.5) | 4 (5) | 22 (27.5) |
| Imipenem | 68 (85) | 2 (2.5) | 10 (12.5) |
| Meropenem | 64 (80) | 2 (2.5) | 14 (17.5) |
| Ampicillin/sulbactam | 42 (52.5) | 13 (16.2) | 25 (31.2) |
| Piperacillin/tazobactam | 58 (72.5) | 3 (3.8) | 19 (23.8) |
| Ticarcillin/clavulanic acid | 55 (68.8) | 5 (6.2) | 20 (25) |
| Polymyxin B | 11 (13.8) | 6 (7.5) | 63 (78.8) |
aValues are expressed as No. (%).
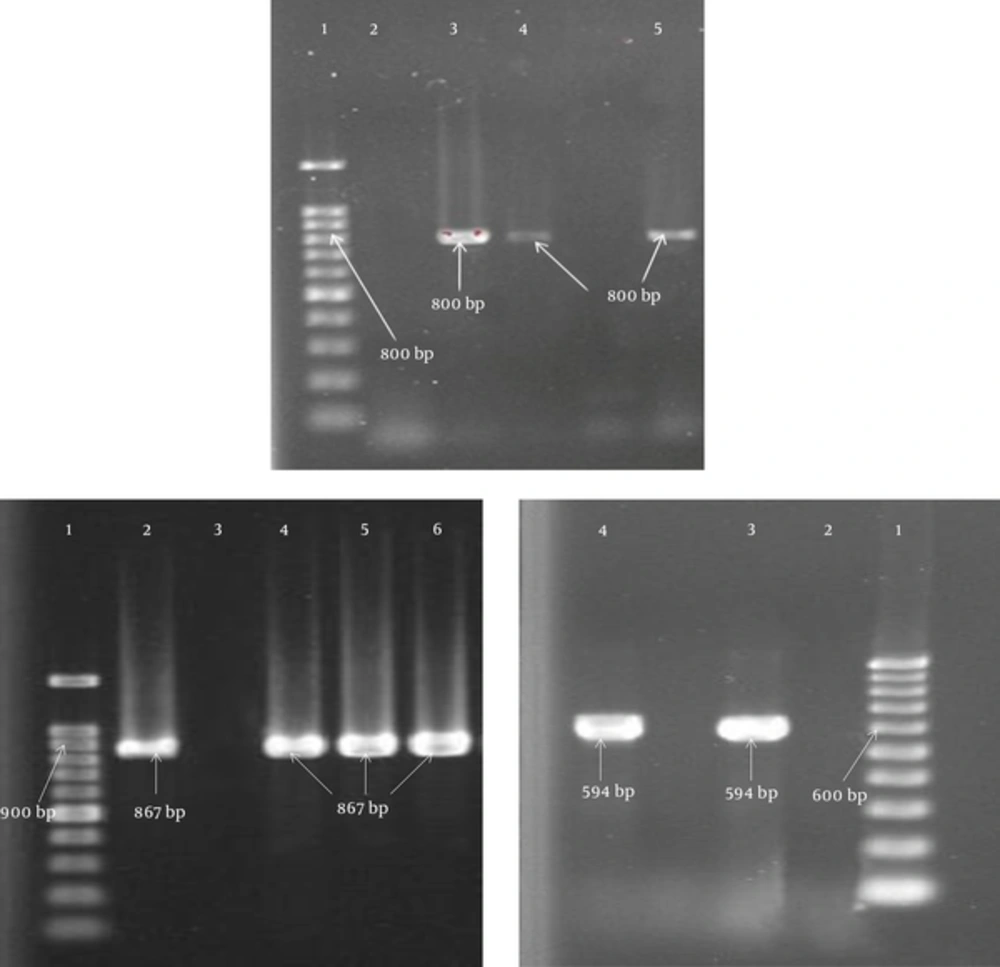
18 (41.9%), 11 (25.6%), and 3 (7%) ESBL-producing isolates expressed SHV, TEM, and CTX-M β-lactamase genes, respectively. The frequency of ESBL genes in the MDR Acinetobacter isolates according to the type of clinical sample is shown in Table 3. There was a statistically significant association between ESBL genes and resistance to some antibiotics. For example, there was a significant relationship between the expression of SHV gene and resistance to cefotaxime (P = 0.038) and tobramycin (P = 0.002). In addition, the expression of TEM gene was associated with resistance to piperacillin/tazobactam (P = 0.021), and the expression of CTX-M gene was associated with resistance to levofloxacin (P = 0.05). The results of PCR assay are given in Figure 2.
| Variables | Tracheal | Urine | Blood | Sputum | Catheter | Wound | CSF | Pleural Fluid | Total |
|---|---|---|---|---|---|---|---|---|---|
| MDR | 19 | 9 | 6 | 6 | 5 | 4 | 1 | 0 | 50 |
| SHV | 8 | 1 | 4 | 0 | 1 | 1 | 0 | 0 | 15 |
| TEM | 6 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 10 |
| CTX-M | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
TEM, 1- Lader (100 bp), 2- Negative control, 3- Positive control (800 bp), 4, 5- Positive sample (800 bp); SHV, 1- Lader (100 bp), 2-Positive control (867 bp), 3- Negative control, 4 - 6- Positive sample (867 bp); CTX-M, 1- Lader (100 bp), 2- Negative control, 3- Positive control (594 bp), 4- Positive sample (594 bp).
5. Discussion
In this study, similar to studies by Farajnia in Tabriz, Iran, and Sinha in India, the majority of isolates of A. baumannii were detected in respiratory samples (15, 16). In contrast, in a study by Vazirzadeh et al., isolates were more commonly obtained from catheter samples (17). Various factors, including consumption of antibiotics, clonal spread of resistant microorganisms, and drug resistance mechanisms of different species, result in the dissemination of highly resistant pathogens to commonly used antibiotics. Previous research reported increased resistance of A. baumannii to various antibiotics and MDR strains hampered the recovery of patients by lowering the efficacy of many common antibiotics (18). The frequency of A. baumannii isolates detected in the present study (62.5%) was in line with that reported by Mirnejad et al., who found that the prevalence of MDR A. baumannii isolates ranged from 32.7% to 93% (19).
In the current study, the highest rate of antibiotic resistance was observed against ceftriaxone (100%), amikacin (96.2%), ceftazidime (95%), and cefotaxime (91.2%), and the highest rate of antibiotic susceptibility was observed against polymyxin B (13.8%). Furthermore, 95.4% of the A. baumannii isolates were resistant to various cephalosporins. This finding is consistent with the results of other studies, which reported that 60% - 100% of A. baumannii isolates were resistant to this class of drugs (19-22). In a study by Kheltabadi et al., only 20% of isolates were resistant to ampicillin/sulbactam (20). In contrast, in the present study, 52.5% of the isolates showed resistance to ampicillin/sulbactam. Due to its ability to inhibit β-lactamases, ampicillin/sulbactam is generally used to treat respiratory and urinary infections. The discord in the findings may be due to differences in the pattern of consumption of antibiotics in this area, as well as increased Acinetobacter resistance to ampicillin/sulbactam.
Carbapenems are one of the most commonly used antibiotics to treat bacterial infections. Hujer et al. reported that only 25% and 20% of A. baumannii isolates were resistant to the carbapenems meropenem and imipenem, respectively (23). However, recent reports pointed to the increased A. baumannii resistance to carbapenems (17, 21, 22), in common with the observations in the present study.
Among various drug resistance-inducing mechanisms, ESBLs play an important role in resistance to commonly used antibiotics, such as penicillin and cephalosporins. Due to the widespread dissemination among nosocomial pathogens via plasmids and integrons, ESBL genes can cause further expansion of drug resistance, including MDR isolates (14, 24). Various studies have reported frequencies of ESBL genes in A. baumannii ranging from 25% to 93.45% (25-28). In the present study, among the 80 examined isolates of A. baumannii, 53.8% were ESBL producers, which is consistent with the results of previous studies. Among the study genes, SHV (41.9%) was the most frequent, followed by TEM (25.6%). Similarly, Safari et al. reported that SHV (58%) and TEM (20%) were the most frequent ESBL genes detected in their study (14). In a study conducted in Iraq, Azhar et al. reported that SHV (25%) was the most frequently detected ESBL gene (29).
In line with the findings of the present research, another study also found that the prevalence of SHV gene was higher in A. baumannii isolates (30). Khalilzadegan et al. reported that CTX-M (19.4%) and TEM (3.2%) were the most frequently detected ESBL genes (21). On the contrary, in the present study, the most frequent gene was SHV. SHV gene is originated from Klebsiella pneumoniae (31). A high frequency of K. pneumoniae bearing SHV gene could likely explain the increased spread and diffusion of SHV gene in bacterial isolates, especially A. baumannii in hospital settings in Kermanshah (32). Various factors can explain the discord in reports on the frequencies of ESBL-producing isolates. These include differences in patterns, especially overuse and misuse, of the consumption of antibiotics; differences in the types of antibiotics used; prolonged hospital stays; use of invasive techniques in diagnoses; and differences in diagnostic methods used to identify genes (33).
6. Conclusion
In this study, A. baumannii isolates exhibited high levels of resistance against the study antibiotics, and a high percentage of the isolates showed MDR. At present, polymyxine B appears to be the only effective antibiotic in the treatment of infections induced by A. baumannii. The frequency of ESBL-encoding genes in A. baumannii isolates may be due to overuse and inappropriate use of antibiotics in the region. To combat overuse and prevent further development of drug resistance in Kermanshah, Iran, more attention should be paid to prescribing practices for various antibiotics, including cephalosporins.