1. Background
Flavonoids are a group of phytogenic polyphenolic compounds, which have various physicochemical characters and chemical structure and are included in many kinds of human diet. In fact, about 4000 compounds of flavonoids have been isolated, and their chemical structures and therapeutic potentials have been investigated (1, 2). Recently, this class of naturally occurring compounds received global scientific attention due to their various therapeutic activities. However, many studies on flavonoids have approved their potentials in treatment of different diseases, such as diabetes mellitus, oxidative stress, allergy, viral infections, cancer, bacterial inflammations, and others (3-9). Naringin flavonoid is a secondary metabolic product found in high amounts in immature citrus fruits as kumquat, pumelo, grapefruit, trifoliate orange, and sour orange, especially in their seeds, peels and in the fleshy parts of their fruits, which give them a bitter taste (10).
Flavonoids have been shown to have a wide range of biological and pharmacological activities in in-vivo and in-vitro studies. Examples include anti-allergic, anti-inflammatory, antioxidant, anti-microbial, anti-cancer, and anti-diarrheal activities (11, 12). Naringin is a natural organic compound, which is also called naringoside, 7-[[2-O-(6-Deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl] oxy]-2,3-dihydro-5-hydroxy-2-(4-hydroxyphenyl)-4H-1- benzopyran-4-one and naringenin 7-O-neohesperidoside. It belongs to the flavanone group of flavonoid glycosides consisting of aglycone part naringenin and glycone part which is a neohesperidose disaccharide. Neohesperidose consists of β-D-glucose and β-D-rhamnose sugar parts, which are responsible for the pharmacokinetic effect of the aglycone part (13).
Many studies have reported that naringin has many pharmacological activities, such as antibacterial (12), antioxidant (14), hypolipidemic (15), anti-hypercholesterolemic (16), anticancer (17), anti-inflammatory (18), and hepatoprotective activities (19). Unfortunately, naringin inhibits some medications metabolizing cytochrome P450 enzymes including, CYP1A2 and CYP3A4, which resulted in various herb-drug interactions (20). Besides, the consumption of naringin can also affect the intestinal absorption of certain drugs, leading to either decrease or increase in circulating drug levels (21).
Hydrazone and oxime derivatives constitute a versatile class of compounds in organic chemistry. The development of novel hydrazone-containing compounds possesses wide verities of biological activities, such as antioxidant, antimicrobial, anticancer, anxiolytic, anticonvulsants, anti-inflammatory, antidepressant, antihypertensive, anti-tuberculosis, and antifungal activity. For that, it was essential to semi-synthesize new hydrazone and oxime derivatives from naringin to improve its pharmacological effects (22, 23).
2. Objectives
The current study aimed at semi-synthesizing hybrid molecules from the natural compound naringin on the bases of hydrazone and oxime derivatives, to estimate their antibacterial activity against some gram-negative and gram-positive bacterial strains and to evaluate their free radical scavenging activity.
3. Methods
All used chemicals in this study were pure and used without any further purification unless otherwise specified; all the chemical solvents and standard compounds used were analytical grades. All the semi-synthesized molecules were identified by 13C NMR, IR, MS and 1H NMR spectroscopy and elemental analysis. The infra-red spectra tests were conducted using Shimadzu 820 PC FT-IR spectrometer, which was recorded in KBr. The NMR spectra tests were screened using the Varian Gemini 2000, 300 MHz and Bruker DPX-300 MHz instruments. Moreover, the 1H NMR tests were established in δ units, parts per million (ppm) downfield from (DMSO) dimethyl sulfoxide (Sigma, Denmark). The 13C NMR spectra was conducted in ppm relatives also to DMSO. The melting points for all the semi-synthesized molecules were tested, utilizing an open capillary tube. Also, thin layer chromatography (TLC) assessment was conducted on plates covered with silica gel and pre-coated with Merck Kiesegel 60 F254, and the obtained TLC results were visualized using a UV lamp. The purifications of the semi-synthesized molecules were conducted by utilizing flash chromatography with silica gel (100 to 200) mesh (24).
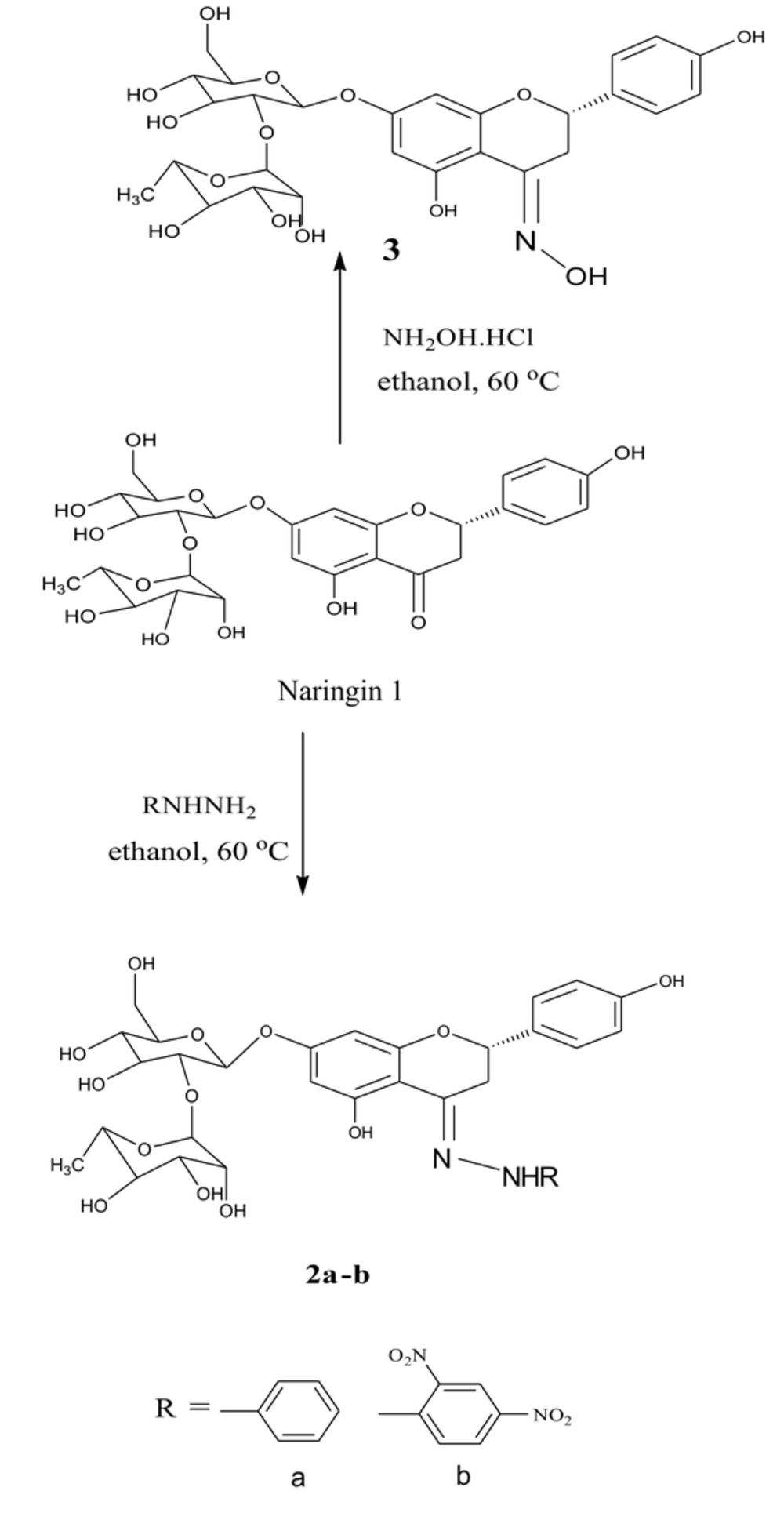
3.1. Preparing for Naringin Based Derivatives
The semi-synthesis method of naringin was carried out by Furniss and Brian method with some modifications and purified using flash chromatography (25). The general steps for semi-synthesis of naringin-based derivatives were started with a 1.5 mmole of the desired hydrazine or amine (Merck & Co., Canada), which were dissolved in 10 mL of ethanol (Fisher Scientific, Ireland) in a round bottom flask. This mixture was acidified with few drops of 3 M HCl solution (IndiaMART, India) then stirred and warmed in a water bath (about 60°C) for ten minutes. Furthermore, 0.87 g of 1.5 mmole naringin (Sigma-Aldrich, Germany) was added to a separate flask and dissolved in 10 ml of ethanol. This suspension was warmed in a water bath until a clear mixture was obtained, which was added directly to the hot solution of hydrazine. The product was slightly heated in a water bath for another 10 to 15 minutes to increase the rate of the reaction. The progression of the reaction was observed using the Thin Layer Chromatography (TLC) method. After the reaction was complete, the reaction mixture was cooled to room temperature and poured into crushed ice with stirring to form the required crystals. Then the mixture was filtered, washed with cold ethanol, and the obtained solids were purified by flash chromatography (ethanol: EtOAc 6:4) (24).
(2S,3R,4S,5R,6R)-2-(((2R,3S,4R,5S,6S)-4,5-dihydroxy-2-(((S,E)-5-hydroxy-2-(4-hydroxyphenyl)-4-(2-phenylhydrazone)chroman-7-yl)oxy)-6-(hydroxymethyl) tetrahydro-2H-pyran-3-yl)oxy)-6-methyl tetrahydro-2H-pyran-3,4,5-triol (2a)
Phenylhydrazine (1.5 mmole, 0.91 mL) was dissolved in 8 to 10 mL of ethanol, which acidified with a few drops of 3 M HCl. The produced mixture was shaked and warmed in a water bath (about 60°C) for 10 minutes. In a separate flask, naringin (1.5 mmole, 0.87 g) was dissolved in ethanol (10 mL). This solution was shaked and slightly warmed in a water bath until a clear mixture was obtained then added to the hot solution of hydrazine. The product was slightly heated in a water bath for another 10 to 15 minutes to increase the rate of the reaction. After that, the mixture was cooled in an ice bath to form crystals, and purified by flash chromatography (ethanol: EtOAc 6:4).
(2S,3R,4R,5R,6S)-2-(((2S,3R,4R,5S,6R)-2-(((S,E)-4-(2-(2,4-dinitrophenyl)hydrazono)-5-hydroxy-2-(4-hydroxyphenyl)chroman-7-yl)oxy)-4,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-3-yl)oxy)-6-methyl tetrahydro-2H-pyran-3,4,5-triol (2b)
Furthermore, 2,4- dinitrophenyl hydrazine hydrochloride (1.5 mmole, 0.355 g) was dissolved in 8 to 10 mL of ethanol, which acidified with a few drops of 3 M HCl solution. The mixture was then stirred and warmed in a water bath (about 60°C) for 10 minutes. In a separate flask, naringin (1.5 mmole, 0.87 g) was dissolved in ethanol (10 mL). This solution was shaked and slightly warmed in a water bath until a clear mixture was obtained, then added to the hot solution of hydrazine. The product was slightly heated in a water bath for another 10 to 15 minutes. After that, the produced mixture was cooled in an ice bath to form crystals, then it was filtered and washed using a small amount of cold ethanol and re-crystallized. The product was purified by flash chromatography (ethanol: EtOAc 6:4).
(S, E)-7-(((2R,3S,4R,5R,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-(((2S,3S,4S,5S,6R)-3,4,5-trihydroxy-6-methyl tetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl) oxy)-5-hydroxy-2-(4-hydroxyphenyl)chroman-4-one oxime (3).
Hydroxylamine hydrochloride (1.5 mmole, 0.11 g) was dissolved in 8 to 10 mL of ethanol, and acidified with a few drops of 3M HCl. The mixture was stirred and warmed in a water bath (about 60°C) for 10 minutes. In separate flask, naringin (1.5 mmole, 0.87 g) was dissolved in ethanol (10 mL), slightly warmed in a water bath until a clear mixture was obtained and then added to the hot solution of amine. The product was slightly heated in a water bath for another 10 to 15 minutes. After completing the reaction, the produced mixture was kept in the bath containing ice to form crystals. Then, it was filtered and washed using cold ethanol and re-crystallized from ethanol. The product was purified by flash chromatography (ethanol: EtOAc 6:4) (26).
3.2. Antioxidant Activity
3.2.1. Chemical Reagents for Antioxidant Test
The chemical reagent 2, 2-Diphenyl-1-picrylhydrazyl (DPPH) was obtained from Sigma-Aldrich (Germany), Trolox (6-hydroxy- 2, 5, 7, 8 -tetramethychroman-2 carboxylic acid) was ordered from Sigma-Aldrich (Denmark), and methanol was purchased from Standard Reagents Pvt. Ltd., (India).
3.2.2. Trolox Standard and Working Solutions
To measure the antioxidant activity, a stock solution with a concentration of 1 mg/mL was prepared in methanol for each of the naringin semi-synthetic derivatives and Trolox (the standard reference) with the following working concentrations, 1, 2, 3, 5, 7, 10, 20, 30, 40, 50, 80, and 100 μg/mL, by serial dilution with methanol from the previously prepared stock solution. Furthermore, the freshly prepared DPPH solution at a concentration of 0.002% w/v (optical density = 1) was mixed with methanol with the above-prepared working concentration in a ratio of 1:1:1, respectively. These working solutions were incubated in a dark location for 30 minutes at room temperature, and the absorbance was recorded at 517 nm, while the spectrophotometer was zeroed using methanol as a blank solution (27). The percentage of antioxidant activity of naringin semi-synthetic derivatives and Trolox standard were calculated using the following formula: Percentage of inhibition of DPPH activity = [(OD517 of Control- OD517 of Sample)/OD517 of Control] × 100. Finally, by using the BioDataFit 1.02 program, the antioxidant half maximal inhibitory concentration (IC50) for the semi-synthesized derivatives, naringin, and the reference compound (Trolox) were estimated.
3.3. Antibacterial Activity
For evaluation of the antibacterial activity, the Micro broth plates and Muller Hilton broth culture media were purchased from Greiner bio-one (North America), while DMSO was ordered from Sigma-Aldrich (Germany).
3.3.1. Microorganisms
In the current study, the used bacterial strains were obtained from the American type culture collection (ATCC), which included Pseudomonas aeruginosa (ATCC 27853), Staphylococcus aureus (ATCC 25923), and Escherichia coli (ATCC 25922) as well as clinical isolate methicillin resistance Staphylococcus aureus (MRSA) strains.
3.3.2. Procedure
At a concentration of 4 mg per 1 mL, the solutions of naringin semi-synthetic derivatives were prepared with 100% DMSO and incubated at 37°C for 24 hours.
3.3.3. Determination of Minimum Inhibitory Concentration (MIC)
The antibacterial potential of the newly prepared naringin derivative was evaluated using the micro-broth dilution method. Briefly, 4 mg/mL of each obtained compound was dissolved in 100% DMSO. Then, these solutions were serially diluted (2-fold) 11 with nutrient broth. Number 11 was considered as the negative control of microorganism growth, while well number 12 contained nutrient broth only and was used as a positive control of the bacterial growth. The final bacterial concentration in each well (except negative control) was adjusted to 5*105 CFU/mL. After inoculation of microorganisms, the plates were covered and incubated for 24 hours at 37°C. Each microorganism isolate was examined in duplicates. The lowest concentration of a derivative solution that did not allow any visible microorganism growth in the test broth was considered as the minimum inhibitory concentration (MIC) (28).
3.4. Antioxidant activity
The antioxidant activity was conducted in triplicates for all the studied samples, and the obtained results were expressed as means ± standard deviation (SD).
4. Results and Discussion
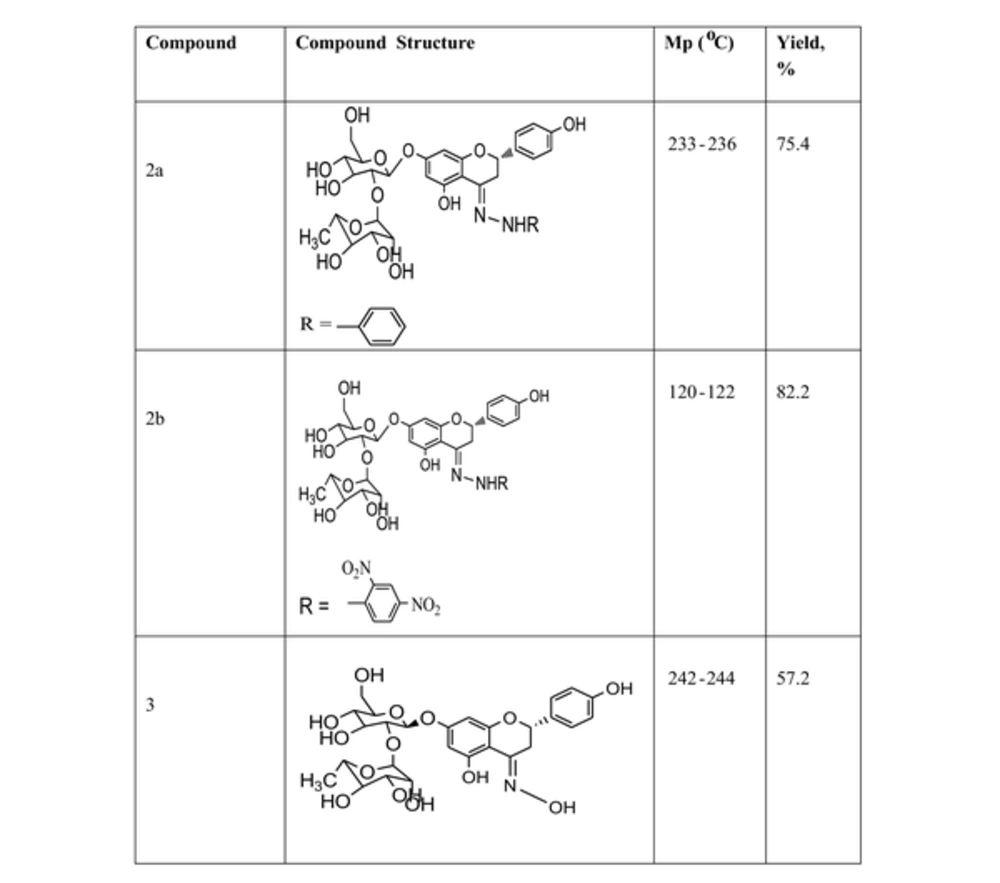
The semi-synthesis of naringin on the bases of hydrazones and oximes produced three novel molecules. Their chemical structures were identified by different spectroscopic and analytical methods, including LC/MS, 1H and 13C NMR, UV, and melting point. All the obtained results were consistently the same with the conventional chemical structures and were in acceptable yields.
4.1. (2S,3R,4S,5R,6R)-2-(((2R,3S,4R,5S,6S)-4,5-Dihydroxy-2-(((S,E)-5-Hydroxy-2-(4-hydroxyphenyl)-4-(2-Phenylhydrazono) Chroman-7-yl)oxy)-6-(Hydroxymethyl) Tetrahydro-2H-Pyran-3-yl)oxy)-6-Methyltetrahydro-2H-Pyran-3,4,5-Triol (2a)
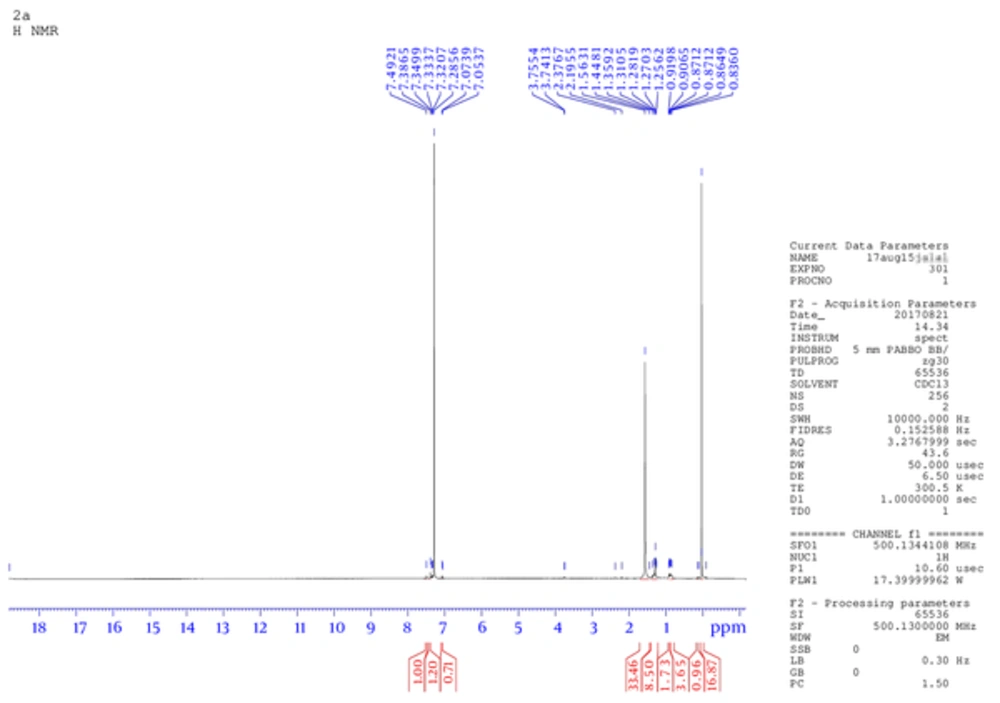
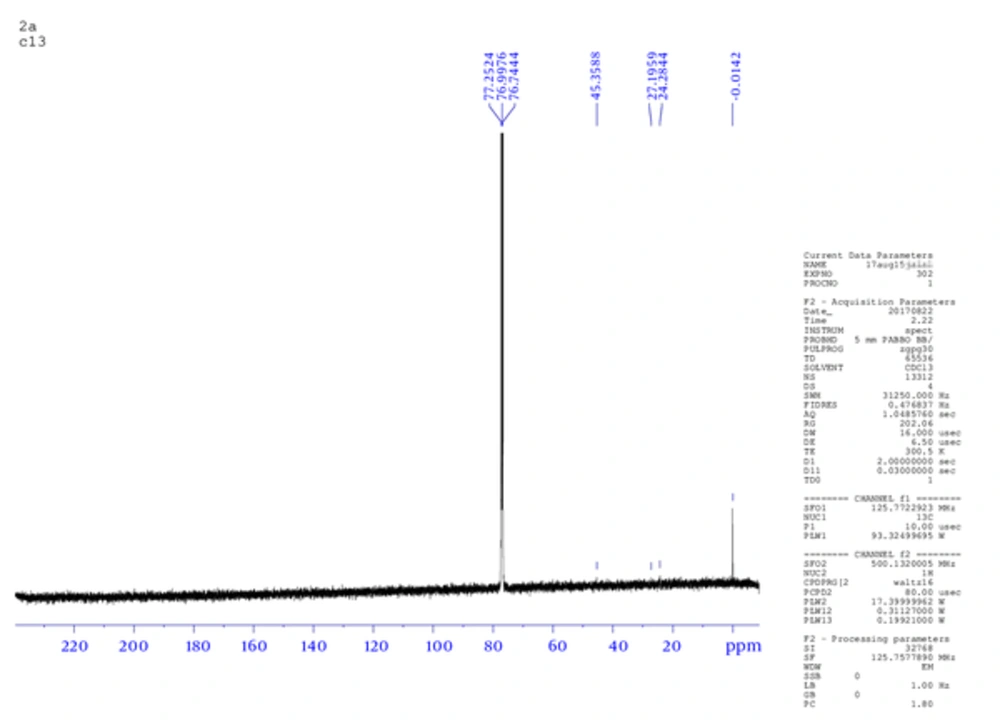
The product weight was 0.56 g (75.4 %), m.p. 233 to 235°C, IR spectra peaks using KBr disk; 3202 (-C - NH), 2990 (-C - OH), 2806 (= CH aromatic), 1600 (-C = N), 1586 (C = C aromatic), 1163 (C - N), and 1077 (C - O ether) cm-1. The Figure 1 shows 1H NMR (300 MHz, DMSO) δ ppm: 2.5 (s, 6 H, OH of sugar), 6.90 (m, 5 H, Ar - H), 7.02 (m, 4 H, Ar -H), 7.27 (m, 2 H, Ar -H), 8.3 (s, 1H, Ph - OH), 10.3 (s, 1 H, Ph - OH). While, Figure 2 is for 13C NMR (DMSO): 39.481, 39.619, 39.756, 39.898, 40.035, 40.176, 40.318, 114.875, 114.970, 121.697, 121.773, 129.271, 129.367, 146.095. MS (TOF- MS) m/z = 670.2 M+ (then 587.32 = M+ - phenol group), theoretical MS = 670. Anal. Calcd for C33H38N2O13: C 59.10, H 5.71, N 4.18, O 31.01.
4.2. (2S,3R,4R,5R,6S)-2-(((2S,3R,4R,5S,6R)-2-(((S,E)-4-(2-(2,4-Dinitrophenyl) Hydrazono)-5-Hydroxy-2-(4-Hydroxyphenyl) Chroman-7-yl)oxy)-4,5-Dihydroxy-6-(Hydroxymethyl) Tetrahydro-2H-Pyran-3-yl)oxy)-6-Methyltetrahydro-2H-Pyran-3,4,5-Triol (2b)
The product weight was 0.62 g (82.2 %), m.p 120 to 122°C, IR (KBr): 3747 (-C - NH), 3399(-C - OH), 2927 (= CH aromatic), 1613 (-C = N), 1539 (C = C aromatic), 1309 (-NO2), 1217 (C-N) and 1052 (C - O ether) cm-1. 1H NMR (300 MHz, DMSO) δ ppm: 2.1 (t, 3H, CH3), 2.5 (s, 6H, OH sugar), 3.3 (s, 1 H, N - H), 7.8 (m, 6 H, Ar - H), 8.35 (d, 1 H, Ar - H containing NO2), 8.37 (d, 1 H, Ar - H containing NO2), 8.38 (d, 1 H, Ar - H containing NO2), 8.8 (s, 1 H, Ph - OH), 10.8 (s, 1 H, Ph - OH). 13 C NMR (DMSO): 16.906, 25.149, 39.041, 39.182, 39.320, 39.461, 39.598, 39.739, 39.877, 115.889, 122.963, 123.005, 128.662, 130.048, 130.086, 136.565, 144.528, 157.550. MS (TOF- MS) m/z = 760.321 M+, theoretical MS = 760. Anal. Calcd for C33H36N4O17: C 52.11, H 4.77, N 7.37, O 35.76.
4.3. (S,E)-7-(((2R,3S,4R,5R,6R)-4,5-Dihydroxy-6-(Hydroxymethyl)-3-(((2S,3S,4S,5S,6R)-3,4,5-Trihydroxy-6-Methyltetrahydro-2H-Pyran-2-yl)oxy) Tetrahydro-2H-Pyran-2-yl)oxy)-5-Hydroxy-2-(4-Hydroxyphenyl) Chroman-4-One Oxime (3)
The product weight was 0.427 g (57.2%), m.p 242 to 244°C, IR spectra peaks using KBr disk: 3735 (OH bonded to N), 3422 (-C - OH), 1699 (-C = N), 1542 (C = C aromatic), 1405 (N - O) and 1017 (C - O ether) cm-1. 1 H NMR (300 MHz, DMSO) δ ppm: 2.5 (d, 3 H, CH3), 2.65 - 2.7 (m, 4 H, OH of sugar), 3.23 - 3.3 (m, 6 H, OH of sugar), 3.4 (t, 1 H, OH of sugar, 5.4 (d, 2 H, OH bonded to N), 6.78 - 6.8 (d, 2H, Ar - H), 7.3 - 7.33 (d, 2 H, Ar - H), 9.5 (s, 1 H, Ph - OH), 10.3 (s, 1 H, Ph - OH). 13C NMR (DMSO): 39.485, 39.623, 39.764, 39.901, 40.043, 40.180, 40.321, 42.440, 78.875, 78.910, 95.386, 95.466, 96.215, 96.295, 102.216, 115.612, 128.771, 128.836, 129.298, 158.189, 163.389, 163.950, 167.096, 196.839. MS (TOF - MS) m/z = 595.4001 M+. Theoretical MS = 595. Anal. Calcd for C27H33NO14: C 54.45, H 5.59, N 2.35, O 37.61.
The characterization data and structures for the semi-synthesized compounds are summarized in Figure 3. Compound 3 was added to the set for comparison, which was prepared from the reaction of naringin with hydroxylamine hydrochloride; the structures and the characterization data for these compounds are shown in Figure 4.
4.4. Antioxidant Activity
The antioxidant activity of newly derived molecules was established and the obtained results were compared with the original compound naringin and with positive control Trolox. The percentage of antioxidant activity of naringin semi-synthetic derivatives and Trolox standard were calculated. The antioxidant IC50 values are reported and explained in Table 1. The results indicated that compound (2a) had the best antioxidant activity with an IC50 value of 3.7 μg/mL. In fact, the (2a) molecule had potential scavenging activity towards free radicals, thus it could be a potent antioxidant compound even when compared with Trolox. Furthermore, the (2b) molecule showed mild antioxidant activity, while compound 3 and naringin, showed weak antioxidant activity with IC50 values of 39.8 μg/mL and 31.8 μg/mL, respectively. In fact, naringin derivatives had conjugated ring structures and several hydroxy groups that had the potential to function as antioxidants in vitro or cell-free systems by scavenging superoxide anion, singlet oxygen, lipid peroxy radicals, and stabilizing free radicals involved in oxidative processes through hydrogenation or complexing with oxidizing species.
| Naringin Semi-Synthetic Derivatives | IC50 Value, μg/mL |
|---|---|
| 2a | 3.7 ± 0.07 |
| 2b | 26.5 ± 0.95 |
| 3 | 39.8 ± 0.1.11 |
| Naringin (N) | 31.8 ± 1.14 |
| Standard (Trolox) | 2.9 ± 0.08 |
aValues are expressed as mean ± SD.
4.5. Antibacterial Activity
The current study investigated the antibacterial activity of three naringin semi-synthetic derivatives to show if they inhibit bacterial growth under 0.25 mg/mL concentration to be considered as effective antibacterial agents. The results of MIC tests for all semi-synthesized derivatives on four bacterial strains are summarized in Table 2. In which compound 3 showed antibacterial activity against P. aeruginosa only with MIC value 0.25 mg/mL, while compound (2b) has MIC value of 0.25 mg\mL against all types of studied bacterial strains. In fact, (2a) derivative showed the lowest MIC value of 0.0625 mg/mL against S. aureus, E. coli, and P. aeruginosa also showed the lowest MIC value of 0.125 mg/mL against MRSA. The obtained results revealed that compound (2a) has the best antibacterial activity than other semi-synthesized compounds. However, it can be considered that phenylhydrazone derivatives of naringin were the most active antibacterial agents.
| Microorganism | 2a | 2b | 3 |
|---|---|---|---|
| S. aureus | 0.0625 | 0.25 | 250 |
| E. coli | 0.0625 | 0.25 | NI |
| P. aeruginosa | 0.0625 | 0.25 | 0.25 |
| MRSA | 0.125 | 0.25 | NI |
Abbreviations: NI, No inhibition of bacterial growth observed at 0.25 mg/mL.
In a study conducted by Mandalari et al. it was shown that naringin has mild antibacterial potential with MIC value of 3 mg/mL against S. aureus and E. coli (29). Other performed studies by Regina et al. confirmed this mild antibacterial activity of naringin against several bacterial strains (30). In fact, hydrazones possess -NHN = CH-, which is very important for the development of new drugs. Both nitrogen atoms of the hydrazone group are nucleophilic. However, the carbon atom of hydrazone group has both electrophilic and nucleophilic activity. Subsequently, hydrazone derivatives’ more particular activity and lower side effect continue to be an active area of intensification in drug discovery (26). Finally, more extensive studies and clinical trials are required to optimize and determine the effectiveness and the mode of action. It is important to investigate other pharmacological activities of the obtained semi-synthesized compounds from the current study.
5. Conclusions
Throughout the current study, three compounds were semi-synthesized by hydrazone and oxime derivatives. Hydrazone (2a) showed a potent antioxidant activity, which was almost the same as Trolox and had antibacterial activity against the four studied bacterial strains. These results can suggest the 2a compound as a potential candidate for manufacturing of potential antibacterial and antioxidant pharmaceutical formulations.