1. Background
Tuberculosis is a contagious disease that is associated with severe complications along with about two million deaths per year (1, 2). Tuberculosis and human immunodeficiency virus (HIV) co-infection together with the high prevalence of drug-resistant Mycobacterium tuberculosis strains furthers complicate the status of the disease (3). Bacillus Calmette-Guérin (BCG) is an attenuated strain of M. bovis that is acquired by repeated passages of M. bovis on potato-glycerol-bile medium for 13 years in 1921 (4). After that year, culture of BCG in different culture conditions and in different parts of the world resulted in the production of several BCG sub-strains with genetic variations and protective characteristics (5, 6). This vaccine can confer protection against severe disseminated form of childhood tuberculosis. However, the major challenge regarding BCG is that it remains to be the only resolution for pulmonary tuberculosis.
Despite many advantages of BCG, this vaccine cannot protect the lungs against mycobacterial invasion (7). Mycobacterium tuberculosis is an intracellular pathogen. Cell-mediate immunity, especially by CD4+ T cells plays a key role in the management of the disease (8, 9). It should be noted that BCG could not be used in immunocompromised patients such as HIV infected patients as they are at high risks for disseminated diseases. Therefore, more effective vaccine, drugs, and diagnostic methods need to be developed to manage this serious disease. Extensive studies have been conducted for this purpose during the past 20 years (3, 10, 11).
Antigen 85a (Ag85a) is a fibronectin-binding protein A (FbpA) that belongs to a complex protein as secretory products of M. tuberculosis. This protein participates in biogenesis of the mycobacterial cell wall (12-14). Ag85a is also involved in several activities such as facilitating the uptake of M. tuberculosis by macrophages, synthesis of trehalose dimycolate (TDM), and maintenance of the cell wall integrity. Experimental studies have shown that Ag85a is a good antigen to induce strong protective immunity against tuberculosis and can be used as a subunit vaccine. Indeed, the capability of Ag85a, in the induction of strong T-cell activation and interferon-γ (IFN-γ) production, has been proven (15, 16).
Cfp-10 is a 10 kDa culture filtrate protein known as early secreted antigenic target protein 6 (ESAT-6) like protein. Cfp-10 is expressed from the region of deletion 1(RD1) region of the mycobacterial genome and has an important role in the pathogenicity of M. tuberculosis (17). Cfp-10 is secreted by the ESX-1 secretion system, which is important for the delivery of the virulence factor Cfp-10 into host cells such as macrophages. Although the specific function of Cfp-10 has remained unclear, every disorder in synthesis or secretion of this protein leads to virulence attenuation of M. tuberculosis and its inability to replicate in macrophages (18, 19). Similar to Ag85a, this antigen is able to promote specific mycobacterial immune responses.
At present, heterologous prime-boost immunization is mostly considered as an effective way for enhancing cell mediated responses in BCG-primed individuals. In this regard, over 9 new vaccines have undergone clinical trials including Ad5Ag85A, Crucell Ad35/ AERAS-402, and M72 + AS01. Among different tuberculosis specific-antigens only a few of them are commonly employed for the development of these vaccines such as Ag85a, Tb10.4, and EsxH. Judgment regarding which vaccines or antigens are better requires completion of clinical trials (20).
2. Objectives
In the present study, a DNA vector harboring Ag85a and Cfp-10 genes constructed previously, s was examined in an animal model to evaluate the ability of the new chimeric protein in stimulating immune responses (21).
3. Methods
3.1. Ethics Statement
All mice were kept in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) (IR.MUMS.REC.1393.160 ethics code) (22).
3.2. Animals
Specific-pathogen-free female BALB/c mice were purchased from the Northeast branch of Razi Vaccine and Serum Research Institute. All mice were four to six weeks old in the beginning of the immunization.
3.3. Construction of Recombinant Plasmid
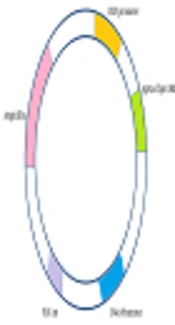
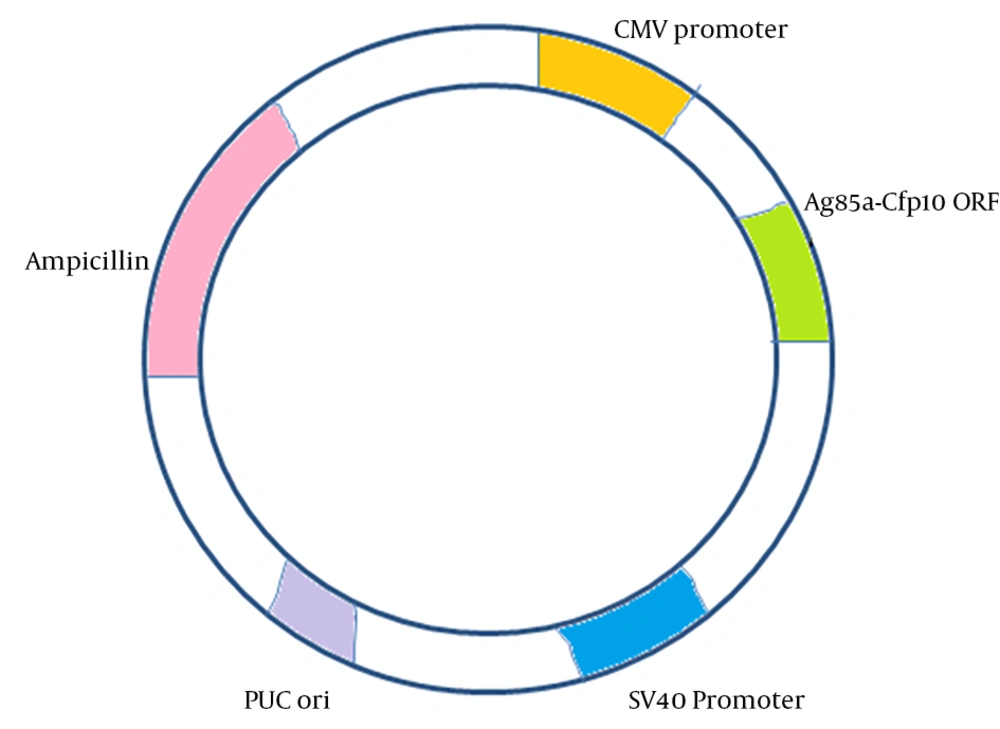
In the previous study, Ag85a and Cfp10 genes were isolated from M. tuberculosis H37Rv genome using specific primers and PCR and was further cloned into pCDNA3.1 (+) as an eukaryotic expression vector. Constructed vector, as shown in Figure 1, was sequenced and showed 100% identity with the recorded genes in GenBank (21).
3.4. RT-PCR
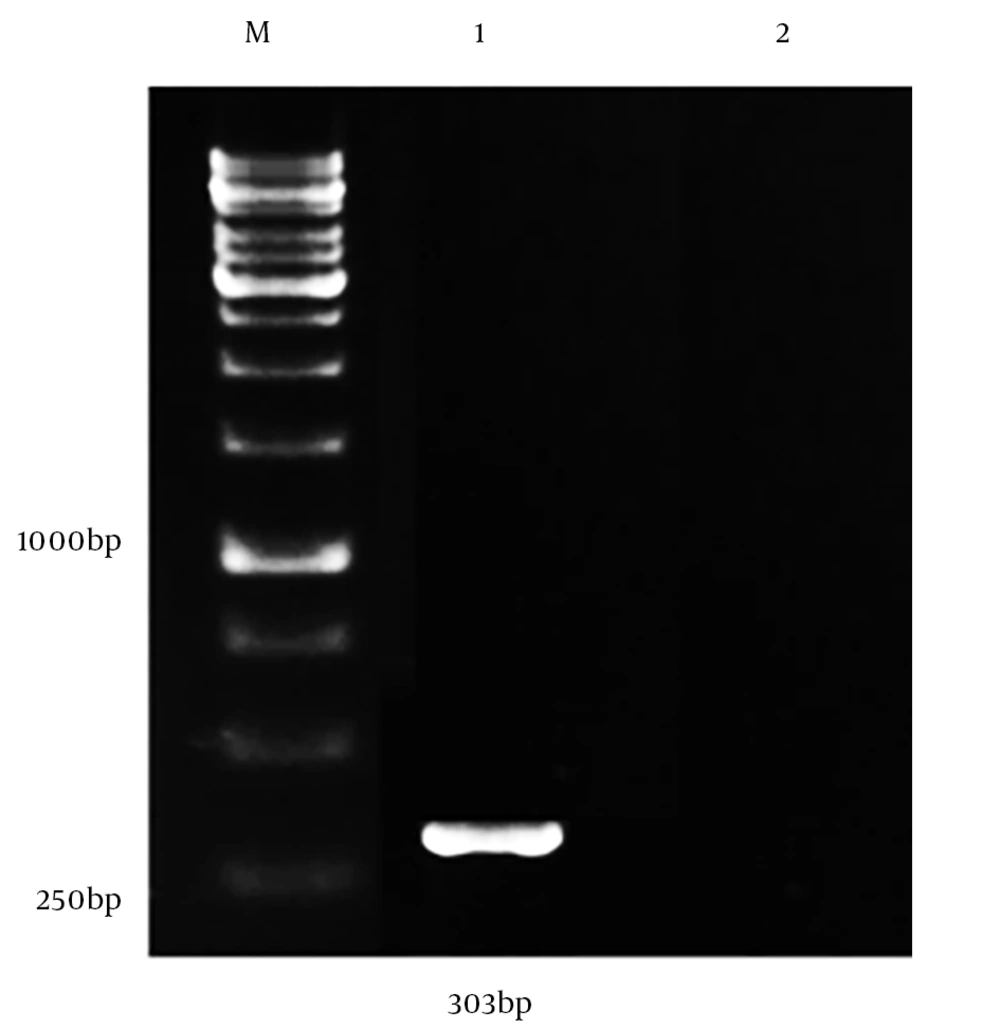
The capability of the constructed vector in the production of Ag85a - Cfp10 fusion mRNA in eukaryotic cells was evaluated by RT-PCR. For this purpose, recombinant Ag85a - Cfp10 -pCDNA3.1+ vector was transfected into Huh-7 (hepatocellular carcinoma) cell line using lipofectamine in accordance with the manufacturer’s instructions (Invitrogen, USA). After the incubation period, transfected cells were recovered and the total RNA extraction was performed using RNX-Plus (SinaClon, Iran), according to the manufacturer’s instructions. The extracted RNA was then applied for cDNA synthesis using the cDNA synthesis kit (Pars Tous, Iran). This was followed by the amplification of cfp-10 using specific primers.
3.5. DNA Vaccination of Mice
An eukaryotic plasmid containing a full length of Ag85a and Cfp10 gene was developed in the previous study (23). To propagate this plasmid, Escherichia coli strain JM109 was transformed with the recombinant plasmid and was purified by the Qiagen kit, based on the manufacturer’s instructions (24). Eighteen female BALB/c mice, at the age of six to eight weeks (25 g) were divided into three groups (n = 6/group): vaccine, BCG, and control groups. The vaccinated group was immunized intramuscularly in both quadriceps with 100 µg purified endotoxin-free recombinant DNA vaccine three times with two week intervals. The control group received sterile saline and the positive group was injected subcutaneously with 5 × 105 cfu of M. bovis BCG vaccine on the first day of immunization. One month after the last immunization, all mice were sacrificed and their splenoctyes were harvested and cultured as described in the previous study (25). Their lymphocytes were harvested from spleen and cultured in enriched RPMI in the presence of 5% CO2 at 37°C (25).
3.6. Immunogenicity
To evaluate the immune responses in mice, cytokines (IL-4, IL-12, IFN-γ, and TGF-β) were measured by ELISA (ebioscience, USA). Thirty days after the last immunization, all mice were sacrificed and their spleen was removed immediately. Then, fresh splenocytes were harvested and cultured in the presence of mycobacterial crude antigens that was obtained from M. tuberculosis lysate in RPMI medium (26). After 72 hours of incubation, the supernatant was collected and the levels of cytokines were measured by ELISA, according to the manufacturer’s protocols (eBioscience, SanDiego, CA).
3.7. Statistical Analysis
Normality of data was analyzed by Kolmogorov-Smirnov test, and one-way ANOVA-tests were used to compare means among groups. A P value of P < 0.05 was considered significant. Analysis was performed with SPSS version 12 (IBM Corporation, New York, USA).
4. Results
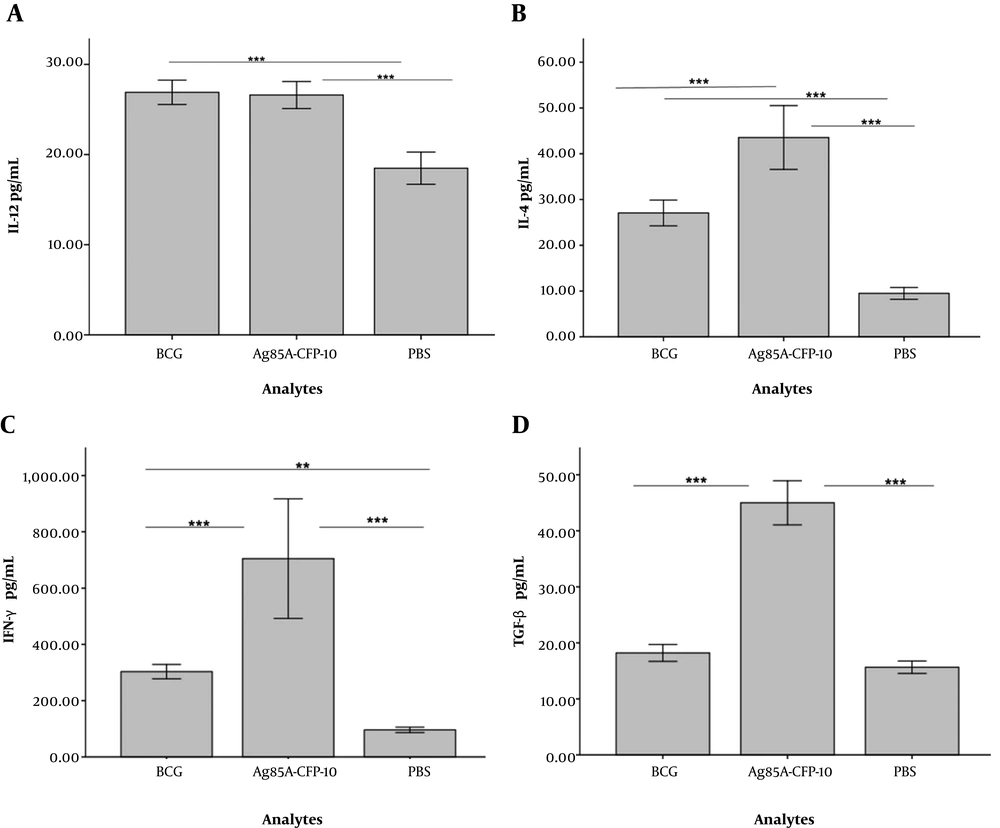
The ability of Ag85a - Cfp10 -pCDNA3.1+ vector in the generation of mRNA was confirmed in the cell culture system using RT-PCR method with amplification of 303bp of cfp-10 gene (Figures 1 and 2) (23). Lymphoproliferation, in response to the antigen, was observed in vaccine and BCG groups while spleen cells from the control group did not proliferate upon stimulation with the antigen. The culture supernatant of splenocytes was collected 72 hours after treatment with antigen. Table 1 shows the level of cytokines, which was assessed by ELISA (eBioscience, USA). The differences between vaccinated and BCG groups were significant in terms of the production of four cytokines (P < 0.001) (Figure 3 and Table 1). The average level of cytokines was measured in the culture supernatant of splenocytes by ELISA.
| Cytokine, pg/mL | PBS | BCG | Vaccine |
|---|---|---|---|
| IFN-γ | 96.28 ± 9.22 | 303 ± 24.32 | 788 ± 24.85 |
| IL-12 | 18.48 ± 1.68 | 26.90 ± 1.28 | 26.60 ± 1.42 |
| IL-4 | 9.48 ± 1.24 | 27.6 ± 2.69 | 435 ± 6.66 |
| TGF-β | 15.65 ± 1.05 | 14.81 ± 1.17 | 45 ± 3.75 |
Measurements of Different Cytokine Levels in BCG, Vaccine and Control Groupsa
Regarding IL-12, differences among BCG (26.90 ± 1.28) and vaccine (26.60 ± 1.42) groups, compared with the control group (18.48 ± 1.68) were significant (P < 0.001). However, there was no statistically significant difference between BCG and vaccinated groups. There were statistically significant differences in the levels of IL-4, among control (9.48 ± 1.24), and BCG (27.6 ± 2.69) compared to the vaccinated (435 ± 6.66) group (P < 0.001). The levels of IFN-γ in the vaccinated (788 ± 24.85) group was significantly higher compared to that of the BCG (303 ± 24.32) group and the control group (96.28 ± 9.22) (P < 0.001). However, the difference between that of BCG group and the control group was not significant (P < 0.01). With regards to the level of TGF-β, there was no significant difference between the BCG group (14.81 ± 1.17) and the control group (15.65 ± 1.05) (P > 0.05). However, the difference between the vaccine group (45 ± 3.75) and the BCG group and the control group was significant (P < 0.001).
5. Discussion
Mycobacterium tuberculosis is an intracellular pathogen. Despite many efforts, it still remains the leading cause of death worldwide. Effective immune responses against intracellular pathogens such as M. tuberculosis, induce cellular immunity. To induce cellular immunity of certain cytokines, and also specific Th1 CD4+ and CD8+, cytotoxic T lymphocyte (CTL) have essential roles. In the tuberculosis infection, activation of macrophages is crucial since this pathogen is able to remain inside the macrophages without any replication, a condition that leads to latent status. Followed by any suppression in the immune system, latent infection progresses to active disease in people who have an asymptomatic or latent infection. Alveolar macrophages are the primary cells targeted by M. tuberculosis. In infected cells, fusion of the phagosome with lysosome and production of reactive nitrogen intermediates (RNIs) is blocked. This allows bacteria to resist at the intracellular environment (27-30).
Previous studies have shown that several cytokines, such as IL-12, interferon-γ (IFN-γ), and TNF-α contribute to the activation of macrophages, enhancing the development of Th1 cells and, finally elimination of the infection. These cytokines induce antimycobacterial activity through the activation of macrophages, formation of granuloma, increase in antigen presentation, and production of effector molecules. The granuloma is a typical feature of tuberculosis and is a strategy used by the host immune system to restrain the spread of infection. Among different immune cells, IFN-γ-producing CD4+ T lymphocytes have central roles since they directly contribute to the generation of granuloma, activation of cytolytic CD8+ lymphocytes, and macrophage. In the present study, the cytokine profile was assessed in mice that received recombinant DNA vaccine and BCG vaccine. IFN-γ is mainly produced by activated CD4+ and CD8+ T cells and NK cells. IFN-γ is an essential cytokine in stimulating protective immune response against intracellular pathogen like mycobacterial infection.
In response to IFN-γ mediated activation of macrophages, presentation of antigens, differentiation of naïve T cell to Th1 subpopulations, generation of antigen specific- CTL, and production of free oxygen radicals and nitric oxide in macrophage (as the effector way for elimination of M. tuberculosis) is dramatically increased. Any mutation and disruption in the IFN-γ related gene and its receptor is associated with increased susceptibility to M. tuberculosis infection, increased severity of the infection, the absence of granuloma formation, and dissemination of tuberculosis infection (31-33).
Interleukin-12 (IL-12) is another important cytokine that has a direct effect on IFN-γ production by T cells and NK cells. IL-12, along with IFN-γ, have a central role in activation of macrophages, generation of antigen-specific Th1 cells, development of protective and specific immune responses against tuberculosis, and ultimately eradication of tuberculosis infection. Based on previous studies, despite the protective role of IFN-γ, IL-4, as a Th2 biomarker, has a contradictory effect on tuberculosis infection (34, 35). In a study, Buccheri et al. confirmed that IL-4 depletion can increase host resistance to tuberculosis infection. This study indicated that treatment of infected mice, with anti-IL-4/IFN-γ, increased formation of granuloma, production of pro-inflammatory cytokines, and decrease in bacterial counts (36-38).
TGF-β and IL-10 are inhibitory cytokines that prevent the activation of T lymphocytes through inhibiting the production of pro-inflammatory mediators including IFN-γ, TNF-α, and IL-12 and reduction of presenting antigens from antigen-presenting cells (APC) s through down-regulation of MHC class II molecules. Therefore, the increase in TGF-β and IL-10 levels are related to mycobacterial survival within host, debilitation of cell-mediated immunity, and finally increased severity of disease. There is a substantial body of evidence suggesting that M. tuberculosis suppress effectual immune responses by inducing the production of IL-10 and TGF-β (39-41).
Ag85a is involved in several activities such as facilitation of the uptake of M. tuberculosis by macrophage, synthesis of trehalose dimycolate (TDM), and finally maintenance of cell wall integrity. Horwitz et al. showed that immunization of naive hosts with purified extracellular proteins of intracellular pathogens such as M. tuberculosis induces strong protective immunity and can be used as a subunit vaccine (15, 16). In a study that was performed by Huygen et al. a DNA vaccine encoding Ag85a was constructed and considered as a simple way to generate protective immunity against M. tuberculosis. In this study, potent humoral and cellular immune responses in vaccinated mice were reported (42).
Furthermore, in a study conducted by Baldwin, challenging with CSU37, a highly virulent clinical isolate of M. tuberculosis, resulted in Ag85a specific CTL, antibodies, and lymphoproliferation responses in animal models previously administrated by DNA vaccines expressing secreted forms of M. tuberculosis Ag85A (43). These two studies supported the idea that Ag85a A, as secreted components of M. tuberculosis, has a high immunogenicity. Despite potent immunogenicity of the Ag85a, a new vaccine based on this antigen was not promising. MVA85A, a modified vaccinia Ankara, expressing Ag85a, which initial research regarding this vaccine showed high levels of induction of long-lasting cellular immunity in combination with the BCG vaccine, however, further results in clinical trials in 2015 were disappointing (14, 44-46).
Comparative genomic analysis has revealed that RD1 genes are present in M. tuberculosis and M. bovis, as virulent strains of mycobacteria, while they are absent in avirulent mycobacteria strains such as i BCG and H37Ra (47, 48). RD1 genes, including Rv3874 and Rv3875, express the 0 Cfp10 and e ESAT6, respectively, which are very important for the virulence of M. tuberculosis. Introduction of these genes to virulent strains elevate the virulence and immunogenicity of the bacterium through modulating the production of TNF-α production from macrophages and macrophage cell death at different stages of the disease (19, 49). In a study, Wu and et al. showed that Cfp-10 has specific epitope restricted to CD8+ T cell (50).
In the current study, the ability of the DNA vaccine encoding two highly mycobacterial immunodominant antigens (Cfp10, Ag85a) was evaluated in BALb/c mice. The ratio of IFN-γ/IL-4 was about 1.5 (29.63 pg/mL); however, in a previous study in which a DNA vaccine encoding Ag85a-Tb10.5 was examined, this ratio was over 20, which imply that Ag85a-Tb10.5 DNA vaccine is more potent in stimulating cellular responses. In addition, it may presume that the combination of Ag85a with Cfp10 may create new antigen with new properties that completely differ with original ones. To address this question more studies are essentially needed.
Further, compared to another our study in which a DNA vaccine containing Mtb32c and HBHA from M. tuberculosis was evaluated, the ratio of IFN-γ/IL-4 was 16. Interestingly, in comparison to our two previous studies, the levels of TGF-β and IL-4 in mice vaccinated with Ag85a-Cfp10 were higher than the BCG group, which implies that the immune system is more shifted towards the humoral immune system. As discussed, the aim of designing a new vaccine against tuberculosis is promoting cell mediated immunity. This means that compared to our previous works, the Ag85a-Cfp10 construct has relatively poor responses in stimulating effective immune responses. However, more studies are required in the future.
DNA vaccine is a suitable and cost-effective way to introduce potent antigens to the immune system and evaluate the capability of these antigens in stimulating the immune system, especially for preliminary studies. However, it has several limitations such as the possibility of its integration into host genome and increase in the risk of cancer and although previous studies have shown that this possibility is low, serious concerns still exists. Another limitation of human DNA vaccine is related to the poor immunogenicity of DNA vaccine. To overcome this problem several strategies have been employed such as codon optimization of gene of interest, use of electroporation for injection, and co-administration of effective adjuvants. Despite these drawbacks, DNA vaccine still remains as powerful means in the field of vaccine research (51).
5.1. Conclusions
The most critical element of immune response against tuberculosis is cell mediated immunity. In the present study, IL-12 and IFN-γ were measured as biomarkers of cellular immunity. High levels of IFN-γ was observed, however, the level of IL-12 was not elevated. There was also the increased level of IL-4 as a Th2 biomarker. The results may indicate that this vaccine cannot potentially activate cell mediate immunity; however, more studies are needed to investigate its effectiveness on restraining tuberculosis and providing effective protection.