1. Background
Severe pulmonary infections can be due to bacterial, viral, or fungal agents. Clinical and radiological features of fungal respiratory infections are nonspecific and overlap with other respiratory tract infections (1). Therefore, a gold standard diagnosis requires isolation and identification of the etiologic agents in respiratory specimens. Early diagnosis and treatment are essential for the management of immunosuppressed patients. Invasive fungal infections are most frequent in immunocompromised patients, such as those receiving immunosuppressive drugs, chemotherapy, those with hematologic diseases, multiple immune defects, chronic obstructive pulmonary disease, and prolonged hospitalization (2-4). The most common site of infection is the respiratory tract system (3, 4). Many fungi can infect the respiratory tract and the most frequent causative organisms (more than 90%) are Candida and Aspergillus species (3). Pneumocystis jirovecii is an opportunistic organism responsible for pneumonia and associated with high morbidity and mortality in immunocompromised patients (5).
The gold standard method for the diagnosis of fungal infections relies on the isolation of etiologic agents by the cultivation of appropriate clinical samples; but the use of molecular detection of fungal DNA may result in the increased sensitivity of non-invasive specimens (6, 7). Pneumocystis jirovecii is a pathogenic fungus in the respiratory tract, which cannot be cultured, and the standard method for its diagnosis is the microscopic examination of stained specimens from the lower respiratory tract using the invasive lung biopsy or bronchoalveolar lavage (BAL) (8). In patients with clinical signs and symptoms of lung disease, a high index of suspicion is required to diagnose fungal infections and unfortunately, the requests for the diagnosis of such infections are limited.
2. Objectives
The aim of this study was to evaluate the frequency of fungi identified in the respiratory tract samples of patients suffering from recurrent lung disorders and suspicious of lung cancer or mycobacterial infections by culture and real-time PCR.
3. Methods
3.1. Ethics Statement
This study was approved by the ethics committee of Prof. Alborzi clinical microbiology research center, Shiraz University of Medical Sciences. The study protocol conformed to the ethical guidelines of the 1975 Helsinki declaration (ethical code 94-12).
3.2. Sample Collection
One hundred and ninety-two BAL and induced sputum samples from 96 patients suspicious for mycobacterial infections or lung cancer with clinical and radiological signs and symptoms of lung diseases were collected between June 2016 and December 2016 from Shahid Faghihi hospital, Shiraz University of Medical Sciences, Shiraz, Iran. The inclusion criteria were recurrent and severe pneumonia and the need for a bronchoscopy examination. In these patients, bacterial infections were not documented by routine sputum or blood cultures. The patients had not received any antifungal treatment but broad-spectrum antibiotics and they were not responsive to these agents. The bronchoscopy examination and BAL collection were part of their treatment course. Samples were examined in pathology and mycology labs. Demographic data and pathology results were collected from the patient’s records.
3.3. Processing of Samples
Bronchoalveolar lavage specimens were centrifuged at 3000 rpm for 15 minutes and pelletable material was washed in distilled water. To liquefy viscous sputum samples, each sample was treated with 0.5% N-acetyl-l-cysteine (Sigma, St. Louis, MO), 0.2N sodium hydroxide (NaOH)/1% sodium dodecyl sulfate, and 5 M potassium acetate (pH 5.0) (Sigma, St. Louis, MO) (9).
3.4. Mycological Examination
All sputum and BAL samples were handled in a class II biosafety cabinet, cultured (0.01 mL) three times on Sabouraud dextrose agar (Merck, Germany) plates with chloramphenicol. The plates were incubated at 30°C for 10 days. The identification of the yeasts was performed by API 20 C AUX (Biomerieux, France), according to the manufacturer’s instructions. The identification of mold was made by macroscopic and microscopic examination of the isolate after lactophenol cotton blue staining.
3.5. Staining of Samples
The microscopic examination of BAL and sputum to identify P. jirovecii was done for all sediments of samples by immunofluorescence staining of cysts, according to the manufacturer’s protocol (Bio-Rad, France). “Five or more oocysts over the whole slide were reported as indicative of Pneumocystis pneumonia infections and one to five fluorescent oocysts as equivocal results.”
3.6. DNA Extraction and Real-Time PCR
DNA was extracted from the specimens using a commercial extraction kit (Invisorb® Spin bacteria DNA Micro Kit, Berlin, Germany), as per the manufacturer’s instructions. To prepare the standard curve of P. jirovecii, the dihydropteroate synthase (DHPS) gene was cloned using the PCR 2.1 vector (Invitrogen, Carlsbad, California, USA). The concentration of the DNA was calculated and expressed by the number of gene copies/µL in 260 nm absorbance. Serial dilutions of DNA in water (107 to 100DHPS copies/µL) were prepared as the standard for quantification. The primers used were the following: forward 5’-GCTTGGTCCAAGTCGCAAAA-3’ and reverse 5’-AGCAGTGCCCCAAATCC-3’. The hybridization probes were VIC-ATTTACAGGGTGTCTTACAGGTGATGTTATGCCAA-TAMRA. Real-time PCR reactions were carried out in duplicate, as described in Alvarez-Martinez (10). Primers and probe were synthesized by BIONEER (Korea). Samples were analyzed by the ABI 7500 sequence detection system (Applied Biosystems, Foster city, California, USA). This was a descriptive study and the collected data were analyzed in SPSS (version 15) using cross tabulation.
4. Results
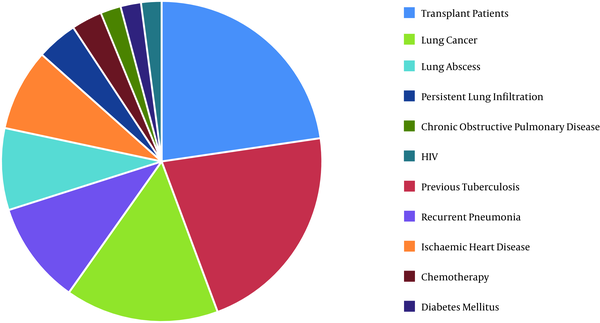
In total, 96 patients were entered into the study. The female to male ratio and the mean age were 25/71 (26%, 74%) and 54 years (SD: 18.3, range 5 to 89 years), respectively. The underlying diseases or predisposing factors at the sampling time are presented in Figure 1.
Fungi identification was successful in 49/96 (51%) patients. The Candida species growth was observed in the culture of BAL and sputum samples of 28/96 (29.2%) patients. The range of Candida colony counts was 0.7 × 103 to > 106 Colony Forming Units/mL. The most frequently identified agents were Candida albicans, C. glabrata, and C. krusei. Aspergillus species were isolated from seven (7.3%) patients, A. flavus from four, and A. fumigatus from three patients.
Pneumocystis jirovecii immunofluorescence staining was positive in 23/96 (23.9%) patients with more than five oocysts and 41/96 (42.7%) patients with less than five oocysts over the whole slide (Tables 1 and 2). By real time-PCR, P. jirovecii was detected in 54.2% of the patients (52/96). The histological examination revealed Mycobacterium genus in nine patients (9.4%) and lung cancer in 10 patients (10.4%). Candida species were isolated from 8/9 (89%) and 8/10 (80%) patients diagnosed with mycobacterial infections and lung cancer (Table 1). Aspergillus species were isolated from one patient with lung cancer. The rates of P. jirovecii in patients diagnosed with mycobacterial infections and lung cancer were 1/9 (11.1%) and 5/10 (50%), respectively. The characteristics of other patients with the fungi identification are shown in Table 2.
| Age/Sex | Diagnosis | Fungal Culture, CFU/mL | Copy Number, P. jirovecii/mL BAL |
|---|---|---|---|
| 78/M | Lung cancer | 2.4 × 103 colonies of Candida albicans | 20 |
| 72/M | Lung cancer | 2.5 × 103 colonies of Candida albicans | Negative |
| 53/M | Lung cancer | 105 colonies of Candida tropicalis | Negative |
| 57/F | Lung cancer | Aspergillus flavus | 456 |
| 68/F | Lung cancer | Negative | 360 |
| 81/M | Lung cancer, HIV positive | > 106 colonies of Candida albicans | 100 |
| 73/M | Lung cancer | 700 colonies of Candida albicans | Negative |
| 75/M | Lung cancer | > 106 colonies of Candida glabrata | Negative |
| 47/F | Lung cancer | > 106 colonies of Candida albicans | Negative |
| 61/M | Lung cancer | 1.5 × 103 colonies of Candida albicans | 100 |
| 65/F | Positive MB3smear | 1.5 × 103 colonies of Candida krusei | Negative |
| 25/M | Positive MB smear | Negative | 120 |
| 28/M | Positive MB smear | > 106 colonies of Candida albicans | Negative |
| 54/M | Positive MB smear | > 106 colonies of Candida albicans | Negative |
| 20/M | Positive MB smear | > 106 colonies of Candida albicans | Negative |
| 75/M | Positive MB smear | 105 colonies of Candida glabrata | Negative |
| 24/F | Positive MB smear | 3.8 × 103 colonies of Candida albicans | 10000 |
| 17/F | Positive MB smear | 2.2 × 103 colonies of Candida albicans | Negative |
| 25/M | Positive MB smear | 5 × 102 colonies of Candida albicans | Negative |
Fungal Species Identified in Patients with Documented Lung Cancer and Mycobacterial Pneumonia
| Age/Sex | Predisposing Factors | Fungi Isolate | Copy Number, P. jirovecii/mL BAL |
|---|---|---|---|
| 32/M | Recurrent pneumonia | > 106 colonies of Candida albicans | 6 × 102 |
| 48/M | Kidney transplantation | Aspergillus fumigatus | Negative |
| 45/F | Lung anthracosis | > 106 colonies of Candida albicans | Negative |
| 49/F | Chronic lymphoblastic leukemia | > 106 colonies of Candida albicans | 1.6 × 102 |
| 63/F | Water pipe smoking | Aspergillus fumigatus | 1.3 × 102 |
| 65/F | Water pipe smoking | Aspergillus flavus | Negative |
| 72/M | Cigarette smoking | > 106 colonies of Candida albicans | Negative |
| 50/F | Water pipe smoking | Aspergillus flavus | Negative |
| 52/M | Cigarette smoking | > 106colonies of Candida albicans | Negative |
| 60/F | Water pipe smoking | > 106 colonies of Candida albicans | Negative |
| 53/M | Water pipe smoking | Aspergillus flavus | 300 |
| 59/M | Kidney transplant, diabetes mellitus | Aspergillus fumigatus, > 106 colonies of Candida glabrata | Negative |
| 52/M | Opium addiction | > 106 colonies of Candida krusei | Negative |
| 65/M | Ischemic heart disease | > 106 colonies of Candida albicans | Negative |
| 58/M | COPD and diabetes mellitus | > 106 colonies of Candida albicans | Negative |
| 65/M | Opium addict | > 106 colonies of Candida glabrata | Negative |
| 51/M | Asthma and pneumonia | > 106 colonies of Candida albicans | Negative |
| 38/M | Unknown | Negative | 3.1 × 103 |
| 59/F | Unknown | Negative | 4.2 × 103 |
| 72/M | Cigarette smoking | Negative | 1.1 × 103 |
| 54/M | Ischemic heart disease | Negative | 8 × 103 |
| 82/M | Recurrent pneumonia | Negative | 8.2 × 103 |
| 69/F | Hypothyroidism | Negative | 2.8 × 103 |
| 67/M | Diabetes mellitus | Negative | 7 ×102 |
| 28/F | HIV | Negative | 5 × 103 |
| 70/M | Diabetes mellitus, myocardial infarction | Negative | 12 × 103 |
| 41/M | HIV | Negative | 1 × 104 |
| 89/F | COPD exacerbation | Negative | 1.44 × 103 |
| 20/M | Minor thalassemia | Negative | 1 × 105 |
Fungal Species Identified in Patients Without Documented Evidence of Lung Cancer and Mycobacterial Pneumoniaa
5. Discussion
Invasive fungal infections in immunocompromised and immunocompetent patients are caused by opportunistic fungi (11). Bacterial pulmonary disease with fungal infection is reported in the literature (12, 13). In this study, fungi were isolated from the samples of patients with recurrent lung disease and not responsive to anti-bacterial agents and receiving no antifungal agents. Bronchoalveolar lavage is the recommended specimen for the diagnosis of fungal elements with high sensitivity of about 50% to 97% (13, 14). It could be a proper and helpful sample in the diagnosis of pulmonary infections. In our study, the mean age of patients was 54 years. According to the literature, older adults undergoing transplantation and aggressive therapy such as immunosuppressive drugs or chemotherapy for malignant or nonmalignant diseases are more susceptible to fungal infections (15).
According to the European organization for research and treatment of cancer/invasive fungal infections cooperative group, and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG), the isolation of Candida species from respiratory secretions is not clinically significant (16). Invasion of lung parenchyma by Candida species is controversial and the isolation of Candida species from the respiratory tract secretions is not clinically significant in many cases and do not need to be treated. However, the isolation is important because in patients with Candida colonization, the rate of systemic candidiasis increases (17). Treatment of all patients colonized with this organism may increase the risk of resistance to antifungal agents, leading to inappropriate costs. In BAL or protected brush specimens, the threshold of 103 or 104 Colony Forming Units/mL of bacteria is accepted to confirm bacterial infections (12). Unfortunately, the criteria for Candida pneumonia have not been defined and the gold standard method to diagnose this infection is the pathologic examination of the lungs biopsy. Histological criteria for the diagnosis of this infection are the presence of pseudohyphae and budding yeasts with acute inflammation (12). The lung biopsy is an invasive procedure; therefore, Candida pneumonia remains unrecognized. Meanwhile, Candida growth was observed in 8/9 (89%) and 8/10 (80%) patients diagnosed with a mycobacterial infection and lung cancer, respectively. In our study, C. albicans was the most frequently isolated species, like in other studies (2, 18).
Pulmonary aspergillosis is a severe infection in immunocompromised and critically ill patients, such as those with the chronic obstructive pulmonary disease, mycobacterial infections, lung cancer, or asthma (12). The mortality rate of pulmonary aspergillosis in hematopoietic stem-cell transplant recipients was reported to be 90% (19). According to Table 1 Aspergillus species were isolated from 7 out of 96 patients included in this study: one patient with lung cancer and 6 patients with the unknown disease. The etiologic agents were A. flavus and A. fumigatus, which are reported in other studies, as well (3, 12). According to EORTC/MSG, the isolation of Aspergillus species from the respiratory tract is significant that would be considered as probable invasive aspergillosis in patients with host and clinical criteria (16). The rapid detection and early treatment of this infection are important due to its high mortality rate. Unfortunately, although the prognosis of this infection is invariably poor in patients, the clinicians in this study had a low index of suspicion for such an infection and the fungal examination was requested for none of these patients.
According to the manufacturer of immunofluorescence staining kit used in our study, cysts of P. jirovecii more than 5 were seen in 23 (24%) patients (probably suffering from PCP) and less than 5 cysts in 41 (42.7%) patients (equivocal for this infection). By real-time PCR, 54.2% of the patients had positive results for P. jirovecii. The rates of P. jirovecii in patients diagnosed with mycobacterium and lung cancer were 1/9 (11.1%) and 5/10 (50%), respectively. Pneumocystis jirovecii may cause pneumonia with respiratory failure, which is a potentially life-threatening infection in case of impaired immunity. The major risk factors for this infection are CD4 counts of < 200 cells/µL, malnutrition, transplantation, and corticosteroids therapy (8). Colonization rates by this organism in patients were reported in the literature: patients with lung cancer 21.7%, with kidney transplantation 20.3%, and patients with other lung diseases 7.3% (20). Co-morbidity between P. jirovecii and bacterial pneumonia and mycobacterial infections was detected in the sputum of 16/367 (4.4%) and 12/227 (5.3%) in Namibian patients, respectively (13, 21). Transmission of P. jirovecii is controversial and colonized patients may play the role of reservoir or carrier, as reported in the literature (22, 23).
6. Conclusions
Our data showed that a high frequency of fungi (Candida species, Aspergillus species, and P. jirovecii) was identified in patients with severe and recurrent pulmonary diseases. They may be presented as documented infection or colonization of airways. In immunocompromised or critically ill patients, colonized fungi can lead to invasive life-threatening lung diseases and they may be transmitted to other susceptible patients. Isolated fungi must be interpreted along with the clinical signs and chest X-ray findings. The management of high-risk patients, particularly those with immunocompromised systems, requires special attention to fungi identified from them.