1. Background
Healthcare-associated infections (HAIs) are a leading problem worldwide. These infections are most prevalent among patients with a major surgery, burns, organ failure, cancer, metabolic disorders, or transplantation. Approximately, 1.7 million patients develop HAIs in the United States each year (1), and it is reported that nosocomial infections are associated with a 4-fold higher patient mortality rate, a 3-fold longer hospitalization, and 2-fold higher hospital costs (2). Patients in intensive care units (ICUs) are one of the major target populations for hospital pathogens. Though ICU-acquired infections constitute about half of all HAIs (3), mortality and morbidity rates are much higher in these infections due to both extensive antimicrobial resistance associated with the pathogens and the critical state of the patients (4). Therefore, monitoring ICU pathogens and documenting their antimicrobial resistance are essential to ensure the prompt organization of measures related to preventive, control, and therapeutic actions.
The pathogens causing HAIs and the susceptibility of these organisms to different antibiotic classes can vary among the countries. Even in a certain area, these parameters may show a changing trend by the time. In Europe-wide surveillances covering 200 health-care facilities from 30 countries, increasing predominance of Gram-negative bacteria with multidrug-resistance trend was determined in the last 10 years, despite decreasing frequency of methicillin-resistant Staphylococcus aureus (5, 6). In contrast, in the United States, it was found that three of the top five HAI pathogens were Gram-positive bacteria responsible for almost half of all HAIs, and S. aureus was reported as the first or second most frequent hospital pathogen between 2005 and 2014 (7, 8). On the other hand, results similar to European data were reported from China, India, and Russia after 2013 (9-11).
In a 10-year surveillance performed between 2000 and 2009, it was determined that Gram-negative bacteria became the predominant pathogen of HAIs and carbapenem resistance among these pathogens doubled in a Turkish tertiary care hospital. In that study, researchers also reported that the frequency of S. aureus reduced about four folds, whereas it was a leading pathogen in 2000 (12). In another study, researchers reported that S. aureus was the most frequent pathogen of bloodstream infections in a Turkish neonatal intensive care unit in 2001. However, after five years, that bacterium became the fourth frequent agent following three Gram-negative species, and responsible for only 10% of all cases (13). Nevertheless, there is still data paucity on the current situation of the HAI pathogens and their antimicrobial resistance in Turkey, particularly in the current decade.
2. Objectives
In this study, we investigated the pathogens isolated from ICU-acquired infections and their antimicrobial resistance detected in a 9-year scale from one of the largest university hospitals in Turkey, which had one of the highest ICU-bed capacities. Additionally, we statistically evaluated changing trends among these parameters throughout the study period with considering the infection control measures applied in our facility.
3. Methods
3.1. Ethics Statement
This study was approved by the local Clinical Research Ethics Committee (approve number: 2016/61), and we used the standard consent form.
3.2. Study Design
This study was conducted at the Turgut Ozal Medical Center, a regional referral tertiary care facility located in the eastern side of Turkey. This hospital has a total of 1,140 patient beds, 265 of which are in the ICUs. The following ICUs were included in this study: reanimation ICUs, medical ICU, neurology ICU, neurosurgery ICU, general surgery ICU, organ transplantation ICU, newborn ICUs, pediatric ICUs, and pediatric and adult burn units. We focused on organisms isolated from ICU patients between 1 January 2007 and 30 September 2015 that were accepted as infecting pathogens according to the Centers for Disease Control and Prevention (CDC) criteria (14, 15). We studied a non-repetitive, single strain for each HAI episode.
This database was produced by active, prospective, and patient-based surveillance studies that were performed throughout the investigation period. Antimicrobial resistance determinants were selected as extended-spectrum beta-lactamase (ESBL) production and carbapenem resistance for Escherichia coli and Klebsiella pneumoniae, carbapenem resistance for Acinetobacter spp. and Pseudomonas aeruginosa strains, methicillin resistance for staphylococci, and vancomycin resistance for Enterococcus spp.
After the legislation of infection control was published by the health ministry, the infection control committee of our hospital started an initiative to combat HAIs at our facility to reduce the health and economic burden caused by this problem in 2007. This initiative included a number of actions such as hand hygiene campaigns, enforcement, and standardization of the sterilization and disinfection procedures, and education activities. In this study, we also discussed the possible impacts of this initiative on the profile and antimicrobial resistance of the pathogens detected in our ICUs.
3.3. Microbiological Analyses
The clinical samples including blood, urine, cerebrospinal fluid, sputum, wound swabs, pus, catheter, and tracheal aspirate, obtained from the ICU patients, were inoculated in blood agar, eosin-methylene blue agar, chocolate agar, and sabouraud dextrose agar media (Oxoid Ltd., Hampshire, UK) and incubated at 35°C for 24 - 48 hours. The growing organisms were identified using classical bacteriologic methods and automatized identification systems (Vitek II, BioMerieux, Marcy I’Etoile, France; BD Phoenix, Becton Dickinson and Company, New Jersey, USA).
We evaluated the antimicrobial susceptibility of the strains using the disc diffusion method and analyzed the results according to the recommendations of the Clinical and Laboratory Standards Institute (CLSI) (16). The ESBL production of Enterobacteriaceae was investigated using the double-disc synergy test; the carbapenem resistance of Gram-negative bacteria was investigated using imipenem disc susceptibility, the methicillin resistance of staphylococci was studied using cefoxitin disc susceptibility test, and the vancomycin resistance of Enterococcus spp. was investigated using vancomycin disc susceptibility test according to the CLSI criteria (16).
3.4. Statistical Analysis
The collected data were expressed as numbers (n) and percentages (%). Changes in the frequencies of the pathogens and in their antimicrobial resistance as a function of time were calculated as medians with 25% - 75% ratios, and we compared the results using the Mann-Whitney U test in IBM SPSS Ver-22.0 Statistical software (IBM Corporation, New York, USA). A P value of less than 0.05 was accepted as statistically significant.
4. Results
In total, 48263 patients were hospitalized in our ICUs over the 9 years of the study period, and 4272 HAI attacks were detected in 3437 patients. 3,044 samples from these patients yielded clinically relevant microorganisms. Of these samples, 1117 were respiratory specimens including sputum, bronchoalveolar lavage, and tracheal aspirate; 570 were urine; 81 were catheters; 521 were blood; 567 were wound swabs or pus; 39 were cerebrospinal fluids; and 149 were other clinical samples. Gram-negative bacteria (n = 2494, 81.9%) were the most frequent group of organisms isolated from the clinical samples, followed by fungi (n = 298, 9.7%) and Gram-positive bacteria (n = 252, 8.2%). Acinetobacter baumannii (n = 1060, 34.8%), P. aeruginosa (n = 622, 20.4%), E. coli (n = 340, 11.1%), K. pneumoniae (n = 331, 10.8%), Candida spp. (n = 285, 9.3%), and S. aureus (n = 110, 3.6%) were the leading pathogens isolated from the patients. The distribution of the pathogens as a function of time is listed in Table 1.
| Pathogens | Annual Frequency | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015b | Total | |
| Gm(-) bacteria | ||||||||||
| Acinetobacter spp. | 32 (9.7) | 71 (22.3) | 69 (22.8) | 128 (35.5) | 170 (47.2) | 159 (45.9) | 142 (40.3) | 163 (41) | 126 (51) | 1060 (34.8) |
| P. aeruginosa | 66 (20.1) | 84 (26.4) | 79 (26.1) | 81 (22.5) | 61 (16.9) | 57 (16.4) | 64 (18.1) | 82 (21) | 48 (17.5) | 622 (20.4) |
| E. coli | 55 (16.8) | 50 (15.7) | 43 (14.2) | 45 (12.5) | 31 (8.6) | 32 (9.2) | 27 (7.6) | 35 (8.8) | 22 (8.9) | 340 (11.1) |
| K. pneumoniae | 47 (14.3) | 31 (10) | 27 (8.9) | 41 (11.3) | 36 (10) | 33 (9.2) | 37 (10.5) | 43 (10.8) | 36 (13.1) | 331 (10.8) |
| S. maltophilia | 14 (4.2) | 16 (5) | 21 (6.9) | 9 (2.5) | 1 (0.2) | 0 | 2 (0.5) | 2 (0.5) | 1 (0.3) | 66 (2.1) |
| Enterobacter spp. | 6 (1.8) | 2 (0.6) | 13 (4.3) | 4 (1.1) | 8 (2.1) | 8 (2.3) | 6 (1.5) | 4 (1) | 3 (1) | 54 (1.7) |
| Other | 3 (0.9) | 4 (1.2) | 8 (2.6) | 0 | 2 (0.5) | 0 | 3 (0.7) | 0 | 1 (0.3) | 21 (0.6) |
| Fungi | ||||||||||
| Candida spp. | 27 (10) | 24 (7.2) | 20 (6.6) | 32 (8.8) | 44 (11.9) | 37 (10.6) | 44 (12.5) | 37 (9) | 20 (7.2) | 285 (9.3) |
| Aspergillus spp. | 1 (0.3) | 0 | 0 | 4 (1.1) | 1(0.2) | 0 | 4 (1.1) | 0 | 1 (0.3) | 11 (0.3%) |
| Other | 0 | 0 | 0 | 0 | 0 | 0 | 2 (0.5) | 0 | 0 | 2 (0.1 <) |
| Gm(+) bacteria | ||||||||||
| S. aureus | 36 (11) | 19 (5.9) | 11 (3.6) | 7 (2) | 5 (1.3) | 4 (1.1) | 5 (1.4) | 14 (3.5) | 9 (3.2) | 110 (3.6) |
| Enterococcus spp. | 12 (3.6) | 4 (1.2) | 4 (1.3) | 6 (1.8) | 8 (2.1) | 12 (3.4) | 12 (2.8) | 14 (3.5) | 6 (2.1) | 78 (2.5) |
| Co-NS | 26 (7.9) | 11 (3.4) | 5 (1.4) | 2 (0.5) | 0 | 2 (0.5) | 3 (0.7) | 2 (0.5) | 0 | 51 (1.6) |
| Other | 2 (0.6) | 1 (0.3) | 2 (0.6) | 1 (0.2) | 2 (0.5) | 2 (0.5) | 1 (0.2) | 1 (0.2) | 1 (0.3) | 13 (0.3) |
| Total (n) | 327 | 317 | 302 | 360 | 369 | 346 | 352 | 397 | 274 | 3044 |
Abbreviation: Co-NS, coagulase-negative staphylococci.
aValues are expressed as No. (%).
bData of 9 months.
Acinetobacter baumannii was the most frequent pathogen in pneumonia, catheter infections, sepsis, wound infections, and meningitides. On the other hand, Candida spp. was the most frequent organism found in urinary tract infections. The pathogen distribution according to infection types is listed in Table 2.
| Pathogens | Infection Types | ||||||
|---|---|---|---|---|---|---|---|
| Pneumonia | UTI | Catheter Infection | Sepsis | Wound Infection | Meningitis | Others | |
| Acinetobacter spp. | 568 | 80 | 27 | 149 | 212 | 14 | 10 |
| P. aeruginosa | 303 | 97 | 10 | 51 | 122 | 5 | 34 |
| E. coli | 42 | 134 | 3 | 52 | 87 | 3 | 19 |
| K. pneumoniae | 85 | 87 | 15 | 84 | 48 | 9 | 3 |
| S. maltophilia | 44 | 7 | 2 | 8 | 5 | 0 | 0 |
| Enterobacter spp. | 16 | 8 | 2 | 9 | 16 | 0 | 3 |
| Candida spp. | 7 | 141 | 20 | 71 | 27 | 1 | 18 |
| S. aureus | 47 | 1 | 0 | 31 | 29 | 0 | 2 |
| Enterococcus spp. | 4 | 15 | 2 | 28 | 16 | 4 | 9 |
| Co-NS | 1 | 0 | 0 | 38 | 5 | 3 | 4 |
| Total (n) | 1117 | 570 | 81 | 521 | 567 | 39 | 102 |
Abbreviations: Co-NS, coagulase-negative staphylococci; UTI, urinary tract infection.
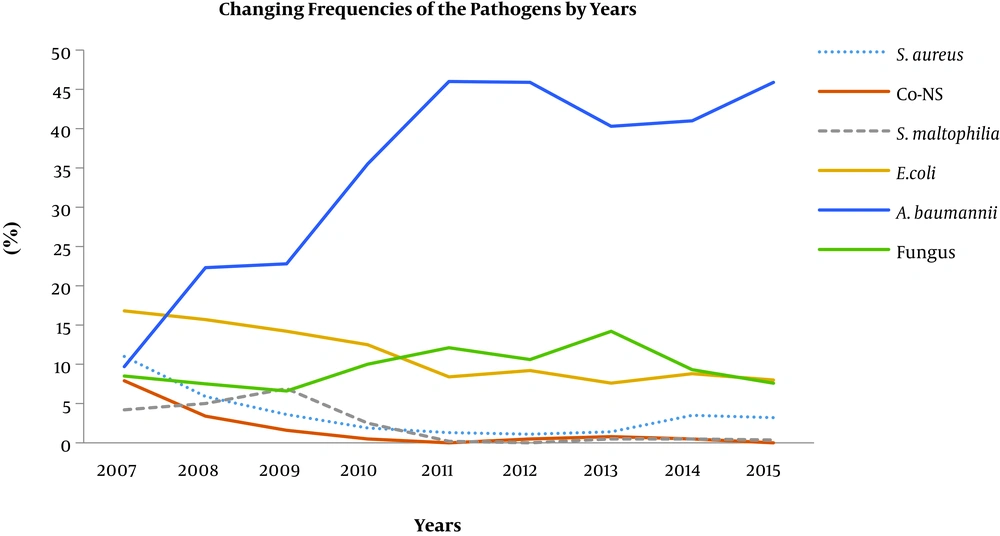
During the study period, statistically significant decreases were detected in the median frequencies of four pathogens. In comparison with 2007 and 2008, the frequency of S. aureus (4.8% vs. 1.4%, P = 0.04) and Co-NSs (5.7% vs. 0.5%, P = 0.04) significantly reduced in the last seven years; and further, in comparison with the 2007 and 2010 period, the frequencies of Stenotrophomonas maltophilia (4.7% vs. 0.3%, P = 0.016) and E. coli (15% vs. 8.4%, P = 0.016) showed substantial decreases in the last five years. In contrast, the rate of Acinetobacter spp. significantly increased (22.6% vs. 45.9%, P = 0.016) in the last six years, and fungi (8% vs. 12.1%, P = 0.03) showed a considerable increase between 2010 and 2013. The changing trends in the frequencies of the pathogens are shown in Figure 1.
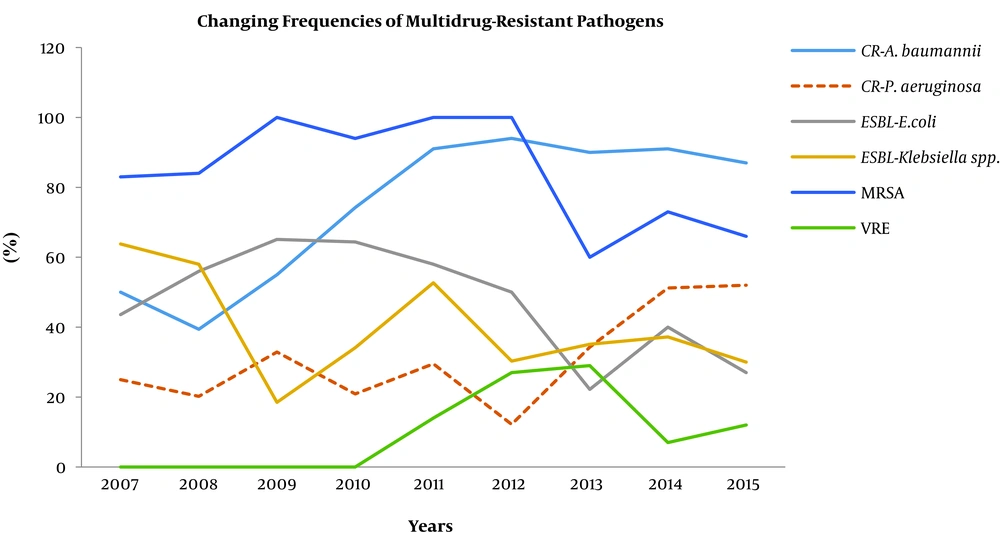
Antimicrobial susceptibility tests revealed that 49.7% of E. coli and 41.3% of K. pneumoniae were ESBL-producing. The rates of carbapenem resistance among these strains were 2% and 9.3%, respectively, and the rates of carbapenem resistance among Acinetobacter spp. and P. aeruginosa were 82% and 30.7%, respectively. In addition, 81.8% of S. aureus and 84.3% of Co-NS strains were methicillin-resistant, and 15.3% of Enterococcus spp. were vancomycin-resistant. We noted a statistically significant reduction in the prevalence of ESBL-producing E. coli (57% vs 27.2%, P = 0.024) over the course of the study. Although a similar decrease was also detected in the ESBL-producing K. pneumoniae, the difference was not statistically significant (52.7% vs 34.2%, P = 0.07).
On the other hand, we observed that the carbapenem resistance in E. coli significantly increased from 0.02% to 12.7% (P = 0.028) in the last two years. In K. pneumoniae strains, the carbapenem resistance increased from 2.6% to 9% (P = 0.016) in the last five years. Similarly, we determined that this resistance also significantly increased in Acinetobacter spp. from 52.5% to 91.4% (P = 0.016) in the last six years and in P. aeruginosa strains, from 25.7% to 51.6% (P = 0.04) in the last two years. The changes in the frequencies of the multidrug-resistant pathogens are illustrated in Figure 2.
5. Discussion
Healthcare-associated infections are the most frequent health problem related to healthcare delivery worldwide. It is reported that 30% of patients in ICUs are affected by at least one episode of HAI in developed countries, and this frequency may be higher by as much as 2 - 3 fold in low-income countries. Furthermore, approximately 140,000 patients die each year due to HAIs in Europe and the United States; the annual cost of this problem in these regions alone is approximately 12 billion Euros (17). Increasing and differentiating antimicrobial resistance are confusing for clinicians for selecting the most appropriate treatment options. However, early initiation of effective treatment is the key determinant for better outcomes from HAIs (18). Therefore, investigating the pathogen profiles and monitoring their antimicrobial susceptibility are valuable for many clinical departments for successful management of ICU patients.
In this study, we observed that a significant fraction (about 85%) of our ICU pathogens were Gram-negative bacteria. These agents showed a regular increasing trend in prevalence throughout the course of the study, and the prevalence of Gram-positive bacteria decreased from 20% to 5.8% among all of the isolates. We think that the reduction in Gram-positive bacteria might have been associated with the initiation of using chlorhexidine-based hand disinfectants in our hospital since 2007. Similar parametric changes, in terms of pathogen profile causing HAI, were determined in a previous Turkish study performed between 2000 and 2009 (12). In contrast, in an earlier Turkish study, S. aureus was found as the leading pathogen of ICU-acquired infections (accounting 30% of all HAIs) between 1995 and 2000, with a regular increasing incidence trend from 10% to 25% throughout the study period (19). On the other hand, we observed that Gram-positive bacteria re-increased in 2014, and we thought that this was possibly due to hiring more than 150 inexperienced nurses to start working in all ICUs by that time. Additionally, the frequency of fungi also increased considerably, particularly after 2010, and they became the second most frequently noted group of organisms in our ICUs. During this time, the number of immune-compromised patients in the liver transplantation, bone marrow transplantation, and cancer treatment units doubled in our hospital; hence, we believe that this increase in the fungi population could be related to the increased number of susceptible patients in our facility.
Recent studies have demonstrated that multidrug-resistant Gram-negative bacteria have been a growing concern for patients in ICUs due to their significant effect on patient mortality (20-22). In a multicenter point-prevalence study conducted in Turkey, researchers reported that Gram-negative bacteria constituted nearly 75% of all ICU pathogens. Furthermore, more than half of Acinetobacter spp. and P. aeruginosa strains were found as multidrug-resistant or extensively drug-resistant (23). In this study, we determined that A. baumannii was the most prevalent organism in our ICUs (34.8%), whereas it was the fifth most frequent pathogen in 2007. The frequencies of P. aeruginosa and K. pneumonia did not change significantly over the course of the study period. On the other hand, the rates of E. coli and S. maltophilia decreased significantly, by up to 16 folds, particularly over the last five years. Interestingly, we observed that the frequency of S. aureus, one of the major nosocomial pathogens a decade ago, decreased by approximately 3 folds from 11% to 3.6% over the course of the study. This result might be one of the most significant outputs of this study, with a high concordance of the results from the current literature (24).
In another study conducted at a Turkish university hospital, researchers reported that nearly 40% - 60% of the E. coli and K. pneumoniae isolates from bloodstream infections were ESBL producers (25). An additional study from Turkey revealed that the frequency of carbapenem-resistant Enterobacteriaceae was 9% and the frequency of carbapenem resistance was 18% among non-fermenter bacilli such as P. aeruginosa and A. baumannii (26). In this study, we determined that almost half of E. coli and K. pneumoniae strains were ESBL-producing. Thought the frequency of ESBL resistance gradually decreased in these species as much as 40% and 50% ratios, respectively, throughout the course of the study, significant increases were detected in carbapenem resistance among four major nosocomial pathogens: E. coli (0.02% to 12.7%), K. pneumonia (2.6% to 9%), Acinetobacter spp. (52.5% to 91.4%), and P. aeruginosa (25.7% to 51.6%) over the same period. Consequently, nearly half (48.6%) of all of the Gram-negative bacteria were found to be resistant to carbapenems in 2015. This finding is possibly the most worrisome outcome of this study because carbapenems are the last group of antimicrobials active against multidrug-resistant Gram-negative organisms.
All of the abovementioned data indicate that the implemented infection control practices and applied antimicrobial use policies in our hospital have provided some benefits on the frequencies of ESBL-mediated resistance, Gram-positive bacteria, and some carbapenem-susceptible Gram-negative bacteria. However, it seems that they have failed to limit the spread of A. baumannii and carbapenem-resistant Gram-negative pathogens in our settings. Furthermore, these results may underline some additional worrisome issues regarding the pathogen selection by antimicrobial use policies in our facility. For example, yet, the increasing use of carbapenems for ESBL-producing organisms has led to a decrease in the infections caused by such strains, but this situation may have caused increasing carbapenem resistance among E. coli and Klebsiella spp. and over-selection of Acinetobacter that is very successful in the rapid development of carbapenem resistance. Therefore, we think that the increasing frequency of carbapenem-resistant Acinetobacter strains in our hospital may be related to the previous high frequency of ESBL producers.
Recent studies have reported that A. baumannii is an increasing problem all over the world. Despite the enforced infection control measures such as those enacted in our hospital, A. baumannii continues to spread in tertiary care hospitals (27, 28). Today, many authors believe that the transmission means of this pathogen are not fully understood, and strong evidence related to the possible airborne spreading of this pathogen is emerging (29). In another study performed in a Turkish tertiary care hospital, the authors reported that the frequencies of top three Gram-negative pathogens including P. aeruginosa, A. baumannii, and E. coli significantly reduced following the reconstruction of the ICUs for acclimatization and staff education (30). Therefore, in addition to enforced infection control measures, providing adequate space and improving the ventilation systems in ICUs can provide positive benefits in combating hospital pathogens.
In this study, we also detected that methicillin resistance in Staphylococcus spp. was rather high in our facility (66% - 100%), but it exhibited non-linear increases or decreases over the course of the study. This rate can be considered very high when compared with the values from other countries. According to the European surveillance, the rate of MRSA was lower than 27% in 24 out of 30 countries, and the continent-wide methicillin resistance rate showed a decreasing trend from 18.1% to 13.7% between 2013 and 2016 (5). Although we recorded a slight increase in vancomycin resistance among Enterococcus spp. between 2011 and 2012, it dropped again in 2013; only three strains were identified in our ICUs in the last three years of the study. Therefore, we think that Enterococcus spp. is not an important HAI pathogen for our facility.
In this study, it was determined that the profile of HAI pathogens changed from Gram-positives to Gram-negatives in our facility, as seen in other tertiary care hospitals in Turkey since 2000. Additionally, we observed that some resistance indicators such as carbapenem resistance in Gram-negatives (particularly for Acinetobacter spp.) and methicillin resistance in S. aureus have reached the highest levels of our country, possibly due to the excessive use antimicrobial agents in our hospital as a result of the fact that a very high number of patients have been treated in our ICUs. We should underline that lacking data belonging to two significant anaerobic HAI pathogens including Bacteroides spp. and Clostridium difficile may be the most important limitation of this study. Furthermore, as a second limitation, this study was performed in a single center rather than a nationwide or multicenter surveillance. However, we think that this study is one of the most important investigations from Turkey in the last decade as the data were collected by a prospective active surveillance covering a large number of isolates in a wide period.
5.1. Conclusions
In this investigation, we documented the surveillance results of searching for ICU pathogens over a 9-year period in a university hospital with one of the largest ICU bed capacities in Turkey. Our work revealed that multidrug-resistant Gram-negative bacteria are one of the predominant pathogens in ICU settings despite infection-control precautions. We believe that this issue is not confined only to the interest area of infection control; this situation ought to be alarming to both health policymakers and many surgical and non-surgical medical departments related to critical care. Therefore, efforts must be increased to fight these organisms that cause difficult-to-care-for infections.