1. Background
Amongst hepatitis viruses, hepatitis B virus (HBV), the prototype member of a family of Hepadnaviridae (1), can cause lifelong infection with a worldwide distribution and is endemic in many populations (2-4). Infection with HBV can lead to a wide range of diseases such as chronic hepatitis, acute hepatitis, asymptomatic HBV carriers, liver cirrhosis and primary hepatocellular carcinoma (HCC) (5-9). The outstanding causes that lead to the development of HCC, the most common type of liver cancer, are hepatitis C virus (HCV) and HBV (5). A study in the south of Iran illustrated that the main factor for HCC was hepatitis B (6). Hepatitis B virus infection is an important public health problem, mostly leading to significant morbidity and mortality in developing countries (7).
Chronic hepatitis B infection is most commonly found in Asia, the Middle East or Africa (8). Although the prevalence of chronic hepatitis in the United States is 25%, HBV is responsible for up to 70% - 80% of chronic hepatitis in Iran, suggesting that HBV infection alone is the outstanding cause of chronic liver disease in Iran (7, 9). Iran is located on the intermediate endemic region for hepatitis B infection and outbreak of HBsAg positivity amongst the general adult population which is approximately 3 to 10 percent in various regions (10, 11). Hepatitis B virus chronic infection is determined by persistence of HBsAg in the plasma for at least 6 months (2, 12). The average time for the diagnosis of HBsAg is 30 days (range: 6 to 60 days) (13, 14). Different factors are involved in the clinical courses HBV infection, including viral factors, immunological factors, genetic host factors and environmental factors (2, 15, 16). Host genetic background is recognized as a considerable factor in patients’ susceptibility to viral hepatitis (11). The natural outcome in HBV infection differs significantly among individuals, so the gene polymorphisms likely determine the outcome of the infection (17).
Several pathways of the cellular immune system during HBV infection are activated including MAPKs, p53, Sex steroid, Wnt/β-catenin, TGF, PI3K/AKT, IKK/NF-κB, Hedgehog and cytokines (4). The understanding of the bases of virus-host interaction necessarily requires expert knowledge of the disease’s immunopathogenesis and its cytokine secretion pattern, as well as the importance of the immunological profile of the host (18, 19).
Interleukin-13, a type 2 T cell helper (TH2), is a pleiotropic cytokine and important regulator of inflammatory immune responses (20-22). IL-13 is a single-chain glycosylated polypeptide that belongs to the IL-13/IL-4 family. IL-13 inhibits production of pro-inflammatory cytokines and chemokines and also down-regulates macrophage activity. Its protein and antibody are more as an important mediator of immunoregulatory processes in various cell types (20, 23). Interleukin-13 is activated in response to the inflammatory diseases. Association of genetic polymorphisms in IL-13 and its receptor component proteins have been verified to be affiliated with higher disease prevalence rates (24).
2. Objectives
This case-control study was designed to evaluate the association of IL-13 gene polymorphisms and the risk of HBV infection. This case-control study was designed to evaluate the effects of TNF-α (-308G > A) and TNF-α (-238G > A) on mRNA expression level and risk of coronary artery disease.
3. Methods
3.1. Subjects
In this study, the blood samples were collected from 585 Iranian subjects including Hepatitis B surface antigen (HBS-Ag) positive individuals who referred to Medical Cellular & Molecular Research Center (MCMRC) of Taleghani Hospital (Gorgan; northeast of Iran) and 283 healthy controls who were recruited from the Golestan Blood Transfusion Organization. The healthy subjects without the history of autoimmune or inflammatory diseases were also matched for age, sex and ethnic origin as HBS-Ag positive cases. According to McDonald’s criteria (25) and based on clinical and paraclinical findings, infectious disease specialists diagnosed HBS-Ag positive cases.
3.2. Nucleic Acid Extraction and Genotyping
DNA was extracted from the whole blood using a modified standard protocol (26). Therefore, red blood cells were lysed in lysis buffer and then SDS (10%), EDTA and 10 μl proteinase K were added to the remaining white cells. By sequence-specific primer-polymerase chain reaction (SSP-PCR) method (27), IL-13 gene polymorphism (+ 110 A or G) was genotyped. Details of used primer sequences and fragment size are presented in Table 1. Each PCR reaction contained 100 - 150 ng of genomic DNA, 9.5 ml master mix containing 20 mm dNTP, 1X Ready load PCR buffer, 12% Sucrose (Merck, Germany), 1U Taq DNA polymerase (GenetBio, Korea), 6 mm human growth hormone (HGH) primer as an internal control, and 30 mm of each specific IL-13 primer.
| Gene | Annealing Temperature | Fragment Primers Size | Sequences |
|---|---|---|---|
| IL-13 | 60 | 204 bp | |
| F (G):5´-ACTTTTTCGCGAGGGACG-3´ | |||
| F (A):5´-ACTTTTTCGCGAGGGACA-3´ | |||
| R (generic):TGAGGTCGGCTAGGCTGA | |||
| HGH | 66 | 429 bp | |
| HGH1: 5´-GCCTTCCCAACCATTCCCTTA-3´ | |||
| HGH1:5´-TCACGGATTTCTGTTGTGTTTC-3´ |
The PCR reaction was carried out in a thermal cycler (Techne, UK). The cycling protocol was included in an initial denaturation at 96°C for 1min followed by 10 cycles of 15s at 96°C, 50s at 66°C, 40s at 72°C (loop 1) and was followed by 20 cycles of 10s at 96°C, 50s at 60°C and 40s at 72°C (loop 2). A total of 30 cycles was carried out with 5min at 72°C as final extension. The PCR products were electrophoresed on a 1.5% agarose gel (Merck, Germany) and stained by ethidium bromide as described previously (28). The genotyping results were tested by the presence or absence of an allele-specific PCR product, which refers to amplification of DNA sequence variants or specific alleles at the same locus. To distinguish between the two alleles, three primers were designed, the generic forward primer, the 3'-A primer and the 3'-G primer. Each allele was identified according to its size (29).
3.3. Statistical Analysis
For Hardy-Weinberg equilibrium, genotype frequencies were evaluated. By using Pearson’s chi-square test and/or Fisher’s exact test, the genotype frequency between HBS-Ag positive cases and control groups was compared. The risk affiliated with genotypes/alleles was assessed as the odds ratio (OR) of 95% confidence intervals (CI). All statistical analysis was done with the Statistical Program for Social Sciences (SPSS version 17.0) software. Differences were considered significant when the P value was less than 0.05.
4. Results
To determine the association of IL-13 gene polymorphisms with HBsAg positiveness, the polymorphic region of IL-13/+110 SNP was investigated in 302 HBsAg positive individuals and 283 control subjects. The distribution of genotypes and allele frequencies of the IL-13/+110 SNP in the case and the control groups are shown in Table 2. The frequencies of A/G genotype (CI = 1.18 - 2.34, OR = 1.66, P = 0.004) and A/A genotype (CI = 0.95 - 3.53, OR = 1.84, P = 0.071) were higher in the patients. As well, the A allele frequency was significantly higher in the patients than the control group (CI = 1.09 - 1.79, OR = 1.84, P = 0.071). However, the present study showed an association between the IL-13/+110 gene polymorphism and HBV.
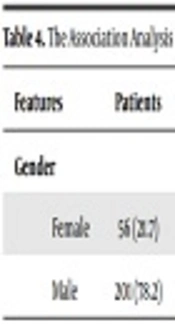
In addition, the frequency of AA/AG/GG genotype in this polymorphic region of IL-13 was investigated in both men and women without considering their health condition (patient or healthy). These results were shown in Table 3. In general analysis, A/G genotype frequency was more prevalent in men and A/A genotype frequency was more common in women while G/G genotype frequency was approximately equal in both sexes. The association analysis of gender subgroups with HBV disease showed most of the subjects were male (Table 4). Statistical analysis showed a significant association between IL-13/+110 gene polymorphism and HBV.
| Il-13 Polymorphism | Controls | Patients | OR | 95% Cl | P Value |
|---|---|---|---|---|---|
| Allele | |||||
| A | 166 (29.3) | 222 (36.7) | 1.40 | 1.09 - 1.79 | 0.007 |
| G | 400 (70.6) | 382 (63.2) | 1 | - | |
| Genotype | |||||
| A/A | 18 (6.4) | 26 (8.6) | 1.84 | 0.95 - 3.53 | 0.071 |
| A/G | 130 (45.9) | 170 (56.3) | 1.66 | 1.18 - 2.34 | 0.004 |
| G/G | 135 (47.7) | 106 (35.1) | 1 | - | - |
| Model of inheritance | |||||
| Recessive (AA vs. AG + GG) | 1.38 | 0.74 - 2.58 | 0.34 | ||
| Dominant (AG + AA vs. GG) | 0.59 | 0.42 - 0.82 | 0.002 | ||
| Co-dominant (AG vs. AA+ GG) | 1.51 | 1.09 - 2.10 | 0.013 |
Abbreviations: OR, odds ratio; CI, confidence interval.
aValues are expressed as No. (%).
| IL-13 Polymorphism | Men, N = 333 | Women, N = 157 |
|---|---|---|
| Genotype | ||
| A/A | 26 (7.8) | 16 (10.2) |
| A/G | 165 (49.5) | 75 (47.8) |
| G/G | 142 (42.6) | 66 (42) |
aValues are expressed as No. (%).
| Gender | Patientsa | Controla | OR | 95% CI | P Value |
|---|---|---|---|---|---|
| Female | 56 (21.7) | 317 (63.1) | 1 | - | - |
| Male | 201 (78.2) | 185 (36.8) | 6.15 | 4.34 - 8.70 | < 0.0001 |
aValues are expressed as No. (%).
5. Discussion
Hepatitis B virus infection is endemic, especially in developing countries that can cause serious liver diseases like hepatocellular cancer, cirrhosis, and chronic hepatitis. Several factors such as host-related factors (e.g. genetic and immunological history), pathogen-related factors (e.g. genotype and viral load), and environmental factors (e.g. nutrition, hygiene, vaccination, and treatment) (30) affect the consequence of HBV infection (31-36). Lot’s of evidence strongly report that host genetic factors (37, 38) may play a significant role in the occurrence of HBV (39) and are therefore widely studied for the various outcomes of HBV infection (16). Hepatitis B surface antigen positivity is more prevalent in identical twins than in fraternal twins (38) indicating that host-related genetic factors will impact on the cycle of HBV infection. Gene polymorphisms can affect the amino acid sequence and alter protein structure and biological function. The attendance of these inherited gene polymorphisms may make a person more resistant or susceptible to a specified disease (36, 40).
The variation in host immune response may be one of the causes of the different clinical representations of HBV infection. Polymorphisms of genes encoding the pro-inflammatory and anti-inflammatory cytokines can affect the infection clinical demonstration. To determine high-risk people for developing chronic hepatitis, the genomic information concerning cytokines and other mediators can be significant and used to plan preventive approaches and treatment for these cases.
Cytokines represent a substantial role in initiating and maintaining a suitable immune and inflammatory response of HBV infection through direct or indirect inhibition of viral replication (15, 41-46). Therefore, several recent studies have paid attention to the effect of gene polymorphisms of cytokines on infectious disease outcome, response to vaccination and treatment. Polymorphisms in genes encoding cytokines may be the primary cause of clinical differences between patients; hence they can be used for the identification of individuals who are at high risk of developing hepatocellular carcinoma and chronic hepatitis (3, 36). So far, we have evaluated the association of several genes with hepatitis and cancer (27, 47-51).
Results of 14 studies of other authors has shown that the rate of HBsAg positive prevalence among 7 provinces of Iran (Golestan, Tehran, East Azarbaijan, Hamedan, Isfahan, Kermanshah, and Hormozgan ) is different and the highest prevalence was reported in Golestan (6.3 percent; 95% CI 3.2 - 9.3 percent) (44, 46). Therefore, consideration of risk factors in this provinceis very important to control the disease. To date, many epidemiological studies have been performed to assess whether polymorphisms in IL-13 are involved in an individual’s susceptibility to cancer. For example, association of decreased risk of glioma with IL-13 rs1800925 polymorphism (52) and IL-13 rs20541 GA and AA variant genotypes (53) were reported. As well as studies showing that cases harboring the IL-13 rs20541 T allele had a reduced risk of colorectal cancer (54) and Chinese tobacco smokers carrying the IL-13 rs1800925 CT variant genotype had 2.57-fold increased risk of bladder cancer (55).
Study of Deng et al. was the first one documenting the relationship between IL-13 genetic variants and hepatitis B virus-related (HBV) hepatocellular carcinoma (HCC). Their study indicated that the functional IL-13 rs20541 polymorphism may contribute to the risk of HCC (56). The human IL-13 gene located on chromosome 5q31 and has effects on immune cells so in this large case-control study, the region of the IL-13 gene position +110 was analyzed via SSP-PCR and gel electrophoresis methods. Amongst the various molecular techniques usually utilized, sequence-specific primer-PCR (PCR-SSP) method is performed as a comparatively easier and cost-effective one. Due to the primers’ specificity due to a 3’ single-base mismatch, this method prevents the priming of a non-specific reaction.
This study showed that the A allele and A/A genotype at the IL-13/+110 position had higher distribution among Iranian HBV patients (36.7% and 8.6% respectively) in comparison to controls (9.3% and 6.4 % respectively). In conclusion, these results suggested that the IL-13 A allele is strongly associated with susceptibility to HBV infection. On the other hand, given the high frequency of G allele in healthy individuals, suggesting that this allele has a protective role in this disease. Nevertheless, our study provides evidence that IL-13/+110 polymorphism may contribute to the risk of HBV, it may vary in different Iranian populations with the divergent genetic background. Taken together, future studies on different ethnic populations and other polymorphic regions in this cytokine gene would provide a more detailed definition for the contribution of IL-13 polymorphisms in the development of HBV disease.
5.1. Conclusions
The polymorphic region of IL-13/+110 was analyzed using SSP-PCR. The results showed that A/A frequency was higher in patients than control subjects. This suggested that A/A genotype probably plays a role in augmenting of susceptibility to HBV infection risk. In addition, a high frequency of G allele in control and healthy cases, suggests that this allele has a protective role against this disease.