1. Background
In the modern medicine, the development of biomaterial sciences and the use of autologous materials are therapeutic approaches to enhance wound healing. One of the important biomaterials is platelet. It is a reservoir of the growth factors in its alpha-granules, which play an important role in the healing of damaged tissues. Many biomaterial products like platelet-rich plasma and leukocyte- and platelet-rich fibrin (L-PRF) are the second-generation of platelet concentrate for the topical use in the treatment of soft tissue diseases and burns, hard-to-heal wounds, oral and maxillofacial surgery, and implantology (1, 2). In the fresh blood, L-PRF is prepared with leukocytes and a fibrin network after activation. In medicine, the use of platelet-fibrin concentrates is one of the oldest approaches of regenerative tissue (2) and the combination of leucocytes and platelets is an interesting healing biomaterial with microbicidal activity. The cell composition of platelet-rich fibrin includes platelets equal to 97% and leukocytes ± 50% of initial blood (3).
Many surgical processes may fail due to infections and the corresponding inflammation; therefore, prevention is important in the patients' outcome. Infections may be caused by the penetration of microorganisms (bacteria or fungi) into the surgical sites depending on the type of causative agents and host immune system. Candida species are one of the most prevalent colonized organisms in the mouth, gastrointestinal tract, vagina, and skin, which can cause systemic infections with high morbidity in immunocompromised patients (4, 5). Streptococcus mitis is another inhabited organism in the human mouth commonly found in the throat and nasopharynx as a colonized organism causing infection in the immunocompromised patients with moderate or severe clinical diseases (6-8).
Some colonized microorganisms due to surface charge and hydrophobicity are able to make cell-cell interactions and cell surface biofilm. Biofilm formation is associated with increased production of antigens and secondary metabolites, the development of antibiotic resistance, and rates of genetic exchange, which can cause clinical problems. In maxillofacial and periodontal surgeries or during the implantation, these biofilms production act as the major risk factors for infections. The use of anti-infective biomaterials such as L-PRF in surgical sites can help the surgeons to improve the healing of the surgical wound.
Antifungal and bacterial activities against some clinical isolates and ATCC microorganisms are reported in the literature for silver nanoparticles (AgNPs) (9-11). Accordingly, the combination of fluconazole with AgNPs enhances the antifungal activity of this agent (11). However, the potential antifungal effects of L-PRF have seldom been studied.
2. Objectives
The aim of this study was to evaluate the anti-biofilm formation of conventional and modified silver nanoparticle-containing L-PRF membranes on the standard Candida and Streptococcus species.
3. Methods
3.1. Ethics Statement
The study protocol was in accordance with the ethical guidelines of the 1975 Helsinki declaration and approved by the ethics committee of Shiraz University of Medical Sciences (code#14866).
3.2. Preparing Silver Nanoparticles Suspension
Silver nanoparticles suspension was prepared by adding 200 mg of the powder (Sigma, Aldrich, USA) with a particle size of < 100 nm to 1 cc of sterile normal saline and sonicated for 2 minutes at 200 watts in a sonication device (Dr. Heilscher, UP 200H, ultraschall prozessor) (12). The serial dilutions with concentrations of 200, 100, 50, 25, 12.5, 6.25 mg/mL were prepared.
3.3. L-PRF and AgNPL-PRF Preparation
Venous whole blood (19 mL) was collected from 18 healthy male volunteers with the age range of 25 - 35 years. The exclusion criteria in this study were a history of any systemic diseases, smoking, and taking any medication or using anticoagulants within the last three months. For each nanosilver concentration, blood samples were divided into two tubes in sterile conditions, one tube containing 10 mL blood for the preparation of the conventional L-PRF and another containing 9 mL blood plus 1 mL silver nanoparticle suspension for the preparation of the modified L-PRF (AgNPL-PRF). Different concentrations of silver nanoparticles were provided from 20 mg/mL to 0.625 mg/mL in whole blood samples. In each concentration, three blood samples were used and L-PRF and AgNPL-PRF membranes were prepared and compared. The tubes were immediately centrifuged for 12 minutes at a low speed (2700 rpm, Eppendorf 5702 centrifuge, Hamburg, Germany), according to the protocol provided by Dohan Ehrenfest et al. (13). The fibrin membranes containing platelets were formed in the buffy coat part and middle of the tubes between the red blood cells and plasma. The fibrin membranes (Figure 1) were removed and placed on a lattice plate in PRF BOX (Process, France) and pressurized under the metal plate for 10 minutes. Each membrane was aliquoted to 4 mm in length and 2 mm in diameter and used in the study process.
3.4. Evaluation of Antibiofilm Characteristics
Three Candida standard species, Candida albicans PTCC 50027 (ATCC 1023), Candida parapsilosis (ATCC 22019), Candida glabrata (ATCC 2001), and Gram-positive Streptococcus mitis (ATCC 49456) were evaluated in this study. The biofilm formations were carried based on Hassan A et al. (14). Candida species and S. mitis were cultured on Sabouraud dextrose agar (Merck, Germany) and blood agar (liofilchem, Italia), respectively. Organism suspensions with a density equal to 0.5 McFarland standards were prepared in fresh RPMI1640 (Sigma, St. Louis, Missouri). 0.5 McFarland standard was equal to 1 - 2 × 108 CFU/mL for S. mitis and 1 - 2 × 106 CFU/mL for Candida species. Three aliquots of different concentrations of AgNPL-PRF and L-PRF from each donor were incubated with 1 mL of 0.5 McFarland suspensions of the entire tested microorganisms at 37°C. After 24 hours, supernatants were aspirated carefully and the membranes were washed twice with phosphate buffer saline (pH 7.3) in order to remove unattached cells. Only the microbial biofilm formed on the clot was evaluated. The biofilm formation of each membrane was evaluated by crystal violet (0.1%) staining (14).
The quality of microorganism’s biofilm formation was evaluated by adding 500 mL of RMPI 1640 medium incubated at 37°C for 24 hours. During this time, the adherent microorganisms started to multiply and scatter daughter cells in the medium. The population of the released daughter cells was evaluated, 200 µL of each medium was poured into 96-well plates, and monitored by an ELISA Reader device (Thermolab system, Multiskan Ascent, Finland) at 490 nm. If the biofilm production of microorganisms was partially or completely inhibited, only a little growth or no growth could be observed. All membranes were tested in triplicate. RPMI medium without any microorganism and 0.5 McFarland microorganism suspensions were considered as negative and positive controls, respectively, and tested under the same conditions as mentioned. Since during the washing process, few microorganisms might be simply attached to the membranes, not to form a biofilm, we defined the index value for this study. An index value equal to one was defined as optical density (OD) of the positive control divided by that of the negative control. The index value for each concentration was calculated by dividing the mean OD of each concentration of membrane by the mean OD of the negative control. Index values were interpreted as negative (< 0.3), positive (= 1), and moderate (0.3 - 0.9).
3.5. Statistical Analysis
Data were collected in SPSS (version 15). Statistical analysis was performed using paired t-test at a significance level of P > 0.05.
4. Results
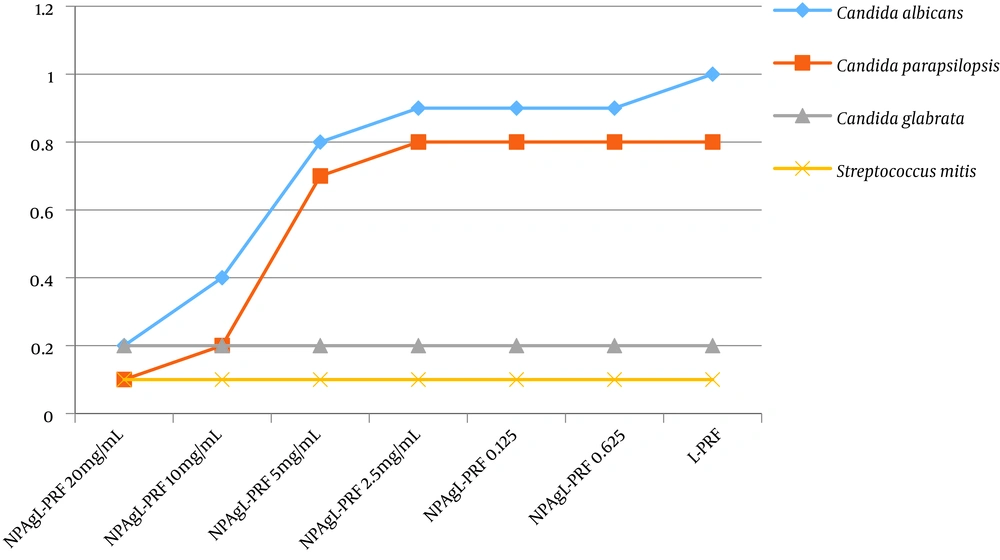
Crystal violet staining showed biofilms around some of the membranes. In general, the inhibition of biofilms formation was in the range of 0.1 to 1 for the L-PRF and AgNPL-PRF in different concentrations depending on the type of microorganism. After 24 hours, no inhibition of biofilm formation in C. albicans was observed in the presence of L-PRF. When using AgNPL-PRF at concentrations of 20 and 10 mg/mL, a significant decrease in biofilm formation was observed. However, no significant differentiation was seen between 10 and 20 mg/mL concentrations of silver nanoparticles in the blood samples. As shown in Figure 2, when the concentration of silver nanoparticles decreased, the growth inhibition was independent of the concentration. The index score for C. parapsilosis in L-PRF showed a moderate inhibition in biofilm formation ability while in AgNPL-PRF with concentrations of 20 and 10 mg/mL, a significant inhibition in biofilm ability was observed, compared to the lower silver nanoparticle concentrations.
Distribution of the anti-biofilm formation for various concentrations in different membranes. The vertical and horizontal lines represent the index value and nanosilver concentration of the membranes, respectively. There is a significant difference between the concentrations in Candida albicans and C. parapsilosis and no significant differences were found in C. glabrata and Streptococcus mitis.
L-PRF and AgNPL-PRF inhibited the biofilm formation in S. mitis and C. glabrata, independent of the silver nanoparticles concentration. For almost all the experimental conditions, the growth inhibition was similar.
5. Discussion
In this study, the antibacterial and antifungal activities of two biomaterials, i.e., L-PRF and AgNPL-PRF, were evaluated against biofilm formation of the standard species of C. albicans, C. parapsilosis, C. glabrata, and S. mitis. We found that in vitro antifungal activities of L-PRF and AgNPL-PRF were not similar and standard L-PRF presents a lower antifungal biofilm formation than AgNPL-PRF in C. albicans and C. parapsilosis.
Healing the surgical wounds is the most desirable procedure in all types of surgeries like implantation in dentistry. During the healing phase, several factors are needed to inhibit the infections and its inflammation and induce cellular proliferation. Platelets are known for their role in the healing of the surgical site due to their growth factors that prevent infection due to antibacterial and fungicidal proteins stored in their granules and blood loss at the site of tissue injury (13). In the families of platelet concentrates, L-PRF contains significant concentrations of leukocytes with a stable dense fibrin matrix and platelet growth factors (15). This fibrin biomaterial represents a new opportunity to improve both the maturation of bone grafts and the final aesthetic result of the peri-implant soft tissue. Therefore, a topical application of L-PRF is used in the treatment of chronic ulcers as an inexpensive, simple, and safe agent for all recalcitrant skin ulcers (16). One of the important roles of L-PRF is the successful dental implant due to the ability to stimulate bone regeneration during dental implants (17). In many clinical trials, the efficacy of this biomaterial has proven for postoperative pain, localized site development, promotion of the soft tissue healing, sinus lift or socket preservation procedures and reduced early adverse effects of the inflammation in surgeries of oral and maxillofacial (18-20).
The use of antimicrobial agents at the site of infections to prevent infections in surgical sites may lead to increased resistant species and intolerable cytotoxic effects of hypersensitivity reactions in the patients. Health care providers should try to minimize spreading resistant etiologic agents by proper management of the antimicrobial use. To do so, the focus should be on the loading of a modified biomaterial like AgNPL-PRF. The antibacterial and antifungal activities of silver nanoparticles have been reported in the literature (21, 22) and enhanced antifungal activity against C. albicans was reported due to the combination of silver nanoparticles and fluconazole (11). The antibacterial properties of silver nanoparticles with bone cement and amalgam were reported in the literature (19, 20). The antimicrobial activity of silver nanoparticle depends on its concentration. Silver nanoparticles showed potent antifungal activity against some Candida species at concentrations of 1–7 µg/mL (9). In the present study, the combination of L-PRF and silver nanoparticles presented similar or more effective anti-biofilm formation in the studied microorganisms compared to conventional L-PRF. The lowest concentration was 10 mg/mL of blood samples. The differentiation in concentrations may be due to the entrapped silver nanoparticles in the fibrin membrane and the sedimentation of some nanoparticles in the tube during the centrifugation of the blood samples and preparation of AgNPL-PRF.
There are populated bacteria and fungi in the human oral cavity, on which the status of oral health depends. Candida species are colonized organisms in some parts of the human body; in immunocompromised patient’s post-surgery, they can pass through the natural body barriers to cause disseminated infections with high morbidity and mortality. Oral candidiasis was diagnosed in 8% of the pediatric patients, and 46.8% of the patients with hematologic disorders were colonized with Candida species (23, 24). Candida albicans is the most commonly implicated organism recovered from 60% of dentate patient's mouth over the age of 60 and 50.5% of Candida species isolated from the oral cavity of liver transplant recipients (25-27). However, other Candida species like C. parapsilosis and C. glabrata may be identified as the cause of infection (28). In this study, L-PRF did not present anti-C. albicans biofilm activity after 24 hours incubation but presented a moderate ability in C. parapsilosis and complete activity in C. glabrata. AgNPL-PRF inhibited the fungal biofilm activity on all studied Candida species, depending on silver nanoparticles concentration.
Streptococcus mitis is a member of the viridans streptococci causing some clinical diseases such as toxic shock-like syndrome, meningitis, and sepsis in immunocompromised patients. It is a normal flora of the human body, like the female genital and gastrointestinal system, oropharynx, and the skin (29, 30). According to the literature, S. mitis is the predominant species in soft tissue surfaces of the oral cavity and maxillary and mandibular lips (7, 8, 30). In this study, two types of the membrane presented anti-S. mitis biofilm formation.
5.1. Conclusions
As revealed, both types of L-PRF presented anti-biofilm formation depending on the type of microorganism. However, AgNPL-PRF biomaterial could inhibit the biofilm formation of all studied microbial populations and it may serve as a more suitable agent for the prevention of tissue infections compared to L-PRF. The lower concentration was 10 mg/mL of blood samples. We suggest that the use of AgNPL-PRF could help the favorable prevention of post-surgery infections in different parts of the human body. This is the first study on modified L-PRF and further research is needed to evaluate the application of this biomaterial.