1. Background
Zika virus (ZIKV) is an insect-borne virus, belonging to the Flaviviridae family, genus, which is similar to dengue fever, Japanese encephalitis, West Nile fever, and chikungunya virus (1). Recently, studies showed that it has Asian and African lineages (2, 3). Many symptoms such as rash, joint pain, and fever were caused by ZIKV, which is similar to many other diseases (3). Nevertheless, ZIKV is not widely concerned due to the fact that symptoms infected with ZIKV were actually asymptomatic (4). However, the outbreak of ZIKV in the northeast of Brazil, in 2015, caused alarming numbers of babies born with microcephalus (5). The Aedes species mosquitoes are the main way, however, the blood, perinatal, and sexual exposures are also allowed ZIKV transmission (2, 6). In 2015, ZIKV was first discovered in Brazil, causing characteristic birth defects, which were associated with microcephaly and Guillain-Barre syndrome. Then, ZIKV was spread rapidly and the large-scale outbreak happened in other continents (7). As of April 2016, there were about 4,800 people who confirmed being infected with ZIKV. Over 46 countries have been reported for the cases of natural ZIKV infections. In China, 13 ZIKV cases have been documented, with the possibility of potential outbreaks still expected. The genetic diversity of ZIKV increased gradually with geographic expansion since 2013 (8). On Feb 1, 2016, ZIKV has been recognized as a public health emergency (9). Recently, it was reported that the ZIKV infection may result in deleterious cardiac effects in some patients (HealthDay News).
Now, ZIKV exhibits the potential to be transmitted to other areas and plausibly results in a future epidemic. Hence, it is needed to detect the virus accurately and rapidly. However, methods for the detection of ZIKV are limited at present (10). The rapid, efficient, and user-friendly test kits are unavailable (11). In addition, there were less reported regarding the highly specific diagnostic reagents for the detection of ZIKV antibodies and antigens. Thus, an antibody based ZIKV fast detection kit would greatly improve the current approaches (12). E (envelope) protein is inlaid on the glycosylated viral envelope protein, which is the key element of the virus surface (13). It determines virulence, virus adsorption, and penetration, which is closely related to the viral disease pathogenesis.
The high N-mannose side chain in E protein plays an important role on the antigenicity of viral proteins (14, 15). Besides, the E protein is a kind of receptor protein, which plays an important role for host range, virulence, and cell tropism. E protein is a main antigen that can produce neutralizing antibody in the process of immune response (16). N-linked glycosylation of E protein is an important factor for ZIKV virulence and neuroinvasion (17). The losing of E N-linked glycosylation leads to compromised expression and the secretion of E ectodomain from mammalian cells (18). This indicates the importance of the E protein. However, it is not clear whether the total length of the E protein can be produced by prokaryotic expression and produced effective epitopes to recognize the ZIKV virion.
2. Objectives
In this work, a prokaryotic expression vector was used to express the ZIKV-E protein. ZIKV-E protein was purified and used as an immunogen to inoculate BALB/c mice (19). Western Blot and indirect immunofluorescence analysis showed that the mouse polyclonal antibody could react with the native E protein in Vero cells specifically infected with ZIKV. The immunoprecipitation, Immuno-LAMP (IC-LAMP), and Immuno-PCR (IC-PCR) technology were used to assess the ability of the polyclonal antibody capturing ZIKV virion.
3. Methods
3.1. Ethics Statement
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
3.2. Virus Strain, Culture Media, Cell Lines and Antibodies
The Escherichia coli host strain DH5α and plasmid vector pET-32a (+) and BL21 (DE3) were purchased from Novagen (Darmstadt, Germany). The ZIKV strain SZ-WIV01 isolated from people in China was amplified in Vero cells, and the supernatant was collected and stored for further use. The viral DNA/RNA extraction kit was purchased from TIANGEN BIOTECH (TIANGEN, China). DMEM medium and FBS were purchased from GIBCO. HRP-conjugated goat anti-mouse IgG antibody (Affinity Biologicals, USA) and goat anti-mouse FITC-conjugated antibody (Abcam, Cambridge, UK) were used in this study.
3.3. Generation of Prokaryotic Expression Plasmid for the ZIKV E Protein
Vero cells infected with ZIKV were cultured in DMEM Media (Gibco™, USA), containing 1% (v/v) FBS. Then, the RNA of ZIKV was extracted using the TIANamp Viral DNA/RNA kit. The extracted RNA was reverse-transcribed into cDNA using HiScript® II 1st Strand cDNA Synthesis Kit (Vazyme, China). The ZIKV-E gene was amplified by PCR with the specific primers F 5' GGAATTCATCAGGTGCATAGGAGTC 3' (underlined, EcoR I site) and R 5' CCGCTCGAGAGCAGAGACGGCTGT 3' (underlined, Xho I site). The EcoR I and Xho I were used to digest the PCR products. Then, the target fragment was gel-purified and ligated into the pET-32a (+) vector to generate the pET-32a (+)-E.
3.4. Expression of the ZIKV-E Protein
The pET-32a/ZIKV-E was transfected into E. coli Rosetta (DE3) cells and cultured in LB liquid medium. Next, 1.0 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to induce the ZIKV-E protein at 20°C for 12 hours (20). The total bacterial lysate was collected and analyzed by SDS-PAGE.
3.5. Purification of the ZIKV-E Protein
The bacteria were collected and washed four times by phosphate buffered saline (PBS; KCl 2.7 mmol/L, KH2PO4 2 mmol/L, NaCl 137 mmol/L, Na2HPO4 10 mmol/L, pH 7.4) and subjected to sonication on ice. The supernatant and precipitation were separated after centrifuged at 10,000 × g, for 20 minutes at 4°C. The precipitations were denatured by 8 M urea (8 mol/L urea, 100 mmol/L NaCl, 50 mmol/L Tris-HCl, pH 7.9) for 3 hours at 4°C. After that, nickel nitrilotriacetic acid (Ni2 + -NTA) agarose resin was used to purify ZIKV-E proteins. Then, purified ZIKV-E proteins were dissolved in PBS buffer for refolding, and determined by SDS-PAGE assay.
3.6. Polyclonal Antibody Production Against ZIKV-E Protein
The purified recombinant ZIKV-E was used to produce antibodies in BALB/c mice. Before immunization, blood was collected from the mice. Sera were collected for negative control purposes after centrifugation. Furthermore, 20 µg ZIKV-E proteins mixed with Freund’s complete adjuvant were emulsified to immunize mice by multipoint subcutaneous injections. Two weeks later, the injections were given with a mixture that contained 40 µg ZIKV-E proteins in each incomplete Freund’s adjuvant. Three days later, after the booster immunization, blood was collected from the mouse. The serum was achieved as described as above.
3.7. Western Blot
The character of anti-ZIKV-E polyclonal antibody was analyzed by Western Blot. The native ZIKV-E proteins from Vero cells infected with ZIKV were used for SDS-PAGE. Then, proteins were transferred to a nitrocellulose (NC) membrane. The NC membrane was blocked with 5% skimmed-milk, which dissolved in the phosphate buffer saline containing 0.05% Tween-20 (PBS-T; KCl 2.7 mmol/L, KH2PO4 2 mmol/L, NaCl 137 mmol/L, Na2HPO4 10 mmol/L Tween-20 22.5 mmol/L pH 7.4) for 2 hours at 37°C. Following that, anti-E polyclonal (1:1000) was added and the NC membrane was incubated for 2 hours at 37°C. After being washed three times by PBS-T, HRP conjugated secondary antibodies (1:5000) were added and the NC membrane was maintained for 1 hour at 37°C. After that, the NC membrane was washed as described above. Finally, the Western Blot Kit Easy See (TransGen, Beijing, China) was used for the detection of the reactivity of the anti-serum.
3.8. Indirect Immunofluorescence Assay
Vero cells were infected with the ZIKV, cultured 36 hours, and fixed 12 hours in pre-chilled acetone-methanol (1/1) for 20 minutes at 4°C. Then, 5% skimmed milk was used to block cells for 1 hour at 37°C. Next, 100 μL antibodies against ZIKV-E (1:1000) diluted in 5% skimmed milk was added and incubated for 2 hours at 37°C. After washing, 100 μL FITC-conjugated goat anti-mouse antibodies (1:2000) was added and maintained for 1 hour at 37°C. Finally, 200 μL PBS was added into 96-well for fluorescence detection at 488 nm using Leica DMI3000B microscope.
3.9. Primer Design
LAMP primers were designed from the nucleotide sequences of ZIKV (GenBank accession numbers MF574579.1) by Primer Explorer V5 software. Five pairs of primers were designed to the homologous regions of ZIKV, and then selected by its amplification efficiency to the target gene (Figure 1).
3.10. The LAMP and PCR Assay
The LAMP reaction containing a set of four primers (F3, B3, FIP, BIP), 320 U/mL Bst DNA polymerase, 2 μL DNA template, 2.5 μL of 10 × LAMP buffer, dNTP mixture, and add water to 25 μL. The mixture was maintained for 50 minutes at 63°C and terminated for 5 minutes at 85°C. The LAMP products were analyzed by separation on 3% agarose gel. Besides, the primers of F3 and B3 were also used for PCR reaction. The reaction was performed in a 25 μL volume containing 1 μL of F3, B3, 12.5 μL of 2 × TSINGKE Master Mix, and genomic DNA. The conditions of PCR reaction contained initial incubation at 95°C for 5 minutes, followed by 35 cycles of 95°C for 30 seconds, 57°C for 30 seconds, 72°C for 30 seconds, and a final extension at 72°C extension for 7 minutes. The products were analyzed in 1% agarose gel by electrophoresis.
3.11. Immunoprecipitation of ZIKV Virion in Cell Culture Medium with Polyclonal Antibody
The immuno-magnetic beads were conducted as described by Zhang et al. (19). A total of 400 μL of mouse anti-ZIKV-E serum (1:100) was transferred into 1.5 mL tubes coated for 0.5 hour at 37°C. The anti-ZIKV-E serum in tubes was removed and washed by PBS-T, and the immuno-magnetic beads were used for capturing ZIKV virion. After that, the mixture containing magnetic beads, antibodies, viruses were used for Western Blot assay and the extraction of cDNA, respectively, under the action of an outside magnetic field. Then, 4 μL of the cDNA was used for PCR, LAMP to verify polyclonal antibody capturing ZIKV. At the same time, the polyclonal antibody of ZIKV-NS1, Shigella, and Salmonella were also used for capturing ZIKV and the cDNA were used as a negative control, respectively.
4. Results
4.1. Generation of the ZIKV-E Protein Prokaryotic Expression Plasmid
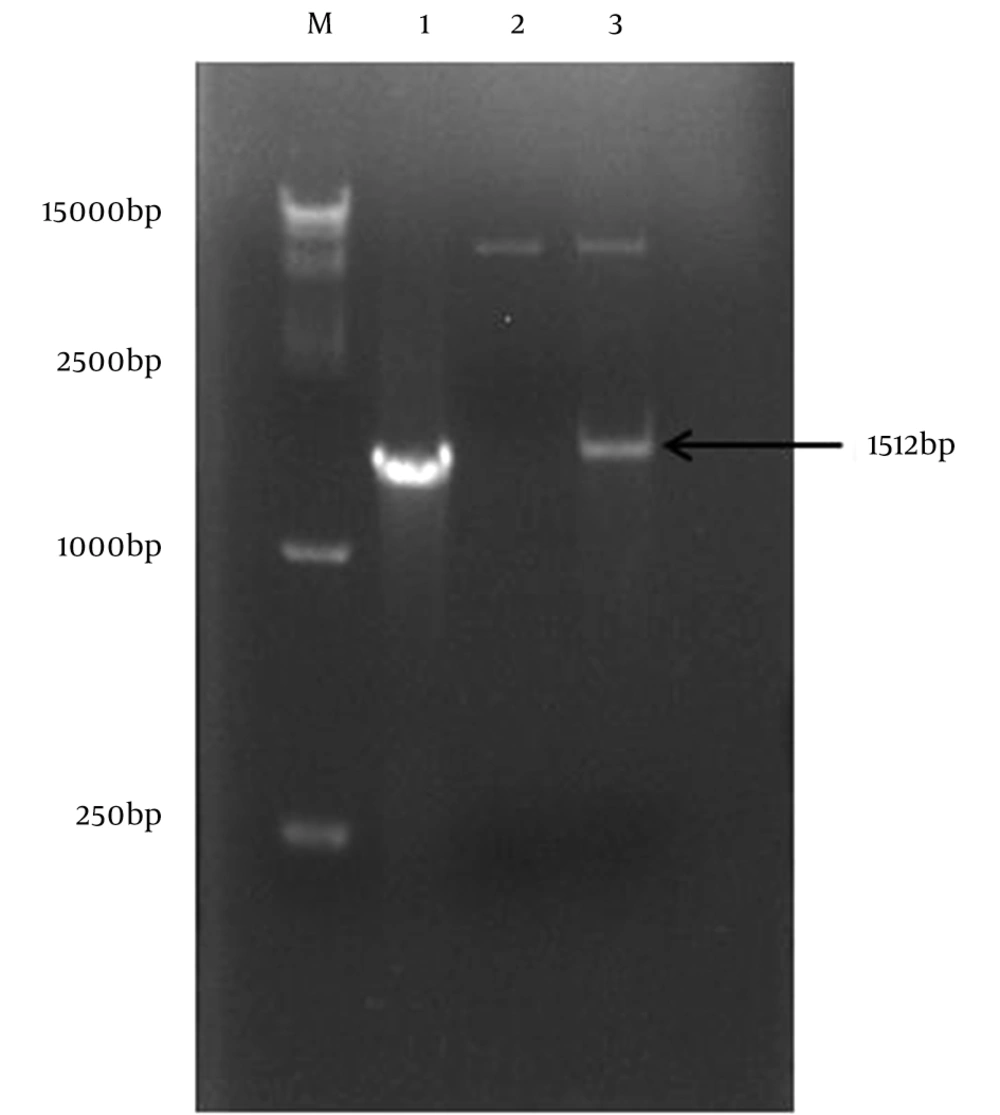
After extract the RNA of the ZIKV SZ-WIV01 strain and PCR reaction, the E protein gene of was amplified successfully (Figure 2, Lane 1). The EcoRI and XhoI were used to digest the PCR product and the fragment was inserted into fraction pET-32a (+) digested with the same enzymes. After that, the recombinant plasmid pET-32a-E was generated successfully. The restriction enzyme digestion and DNA sequencing were used to confirm the plasmid of pET-32a-E (Figure 2, Lane 3). The pET-32a (+) was also by restriction enzyme digestion (Figure 2, Lane 2).
4.2. Expression of the ZIKV-E Protein
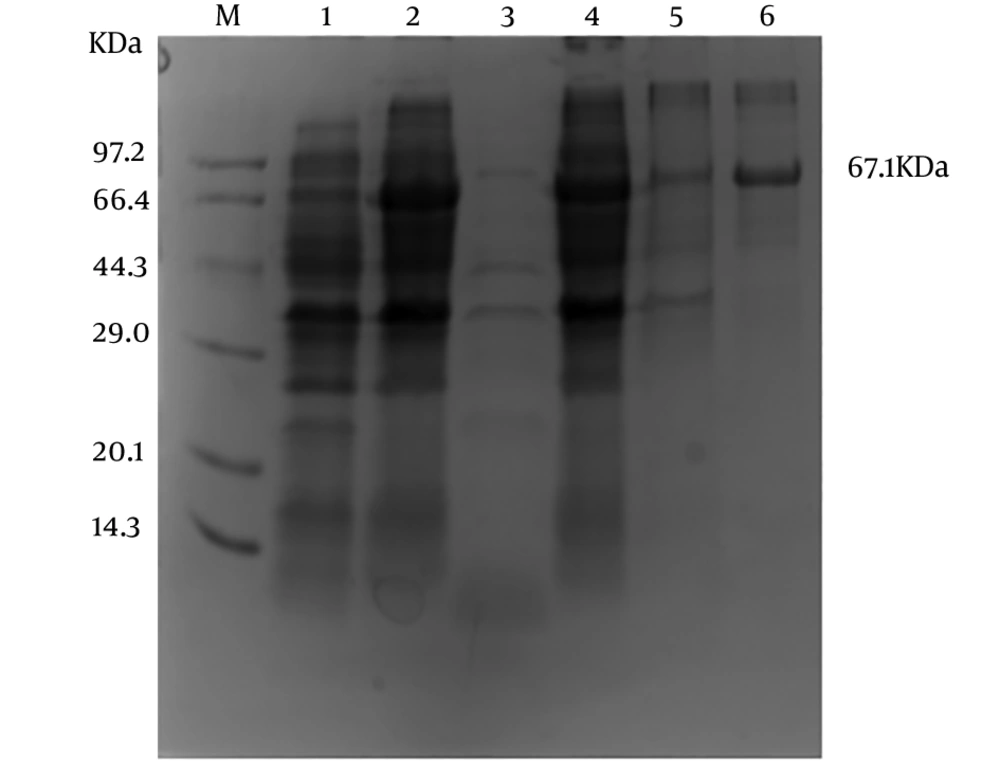
After being inducted with IPTG, the ZIKV-E protein was expressed at a relatively high level (Figure 3, Lane 2). In addition, the ZIKV-E protein was only expressed after induction (Figure 3, Lanes 2 and 4), while there was no expression of the E protein without IPTG induction (Figure 3, Lane 1). The result of SDS-PAGE showed that the majority of the ZIKV-E proteins were expressed in the cell debris pellets (Figure 3, Lane 4), suggesting that the ZIKV-E protein was insoluble as the form of inclusion bodies.
4.3. The Purification of the ZIKV-E Protein
The Ni2+-NTA resin column was used to purify the ZIKV-E protein (14). The result of SDS-PAGE showed that the recombinant ZIKV-E protein could conjugate with Ni2+-NTA resin column, and a single target band about 67.1 kDa was purified (Figure 3, Lane 6). Approximately 1.4 mg ZIKV-E protein was obtained after being determined by the BCA assay.
4.4. Characterization of the Polyclonal Antisera Against ZIKV-E Protein
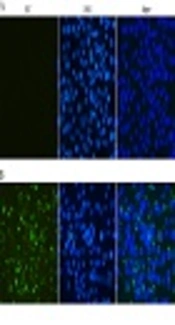
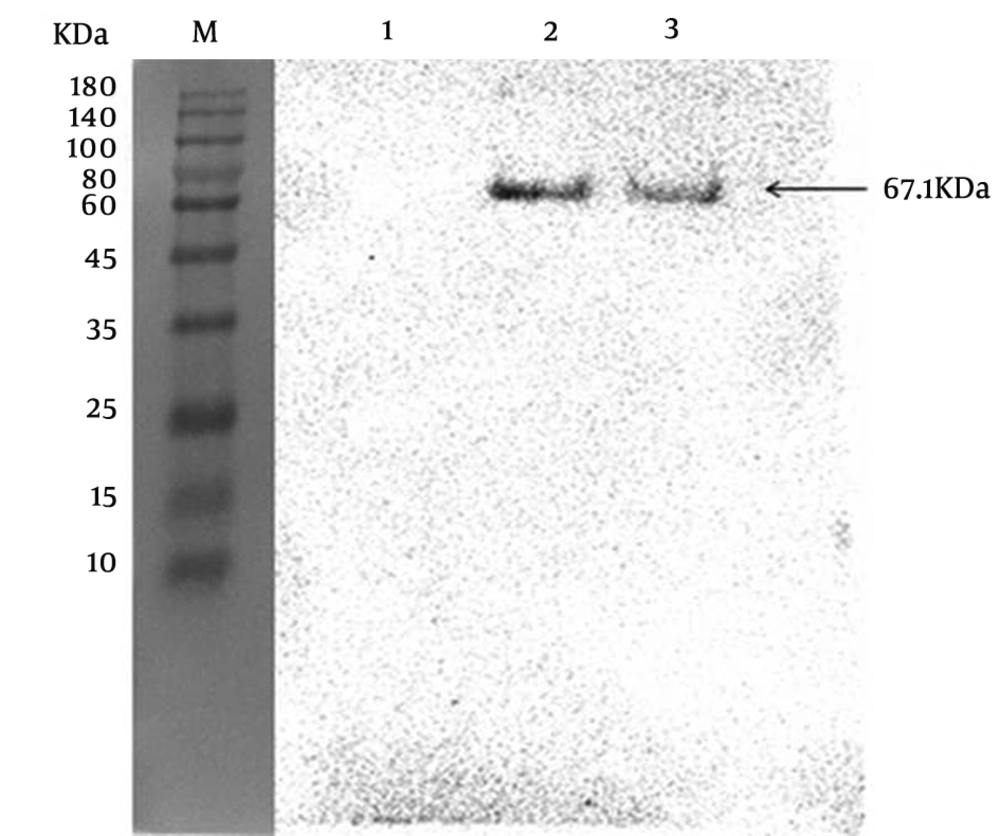
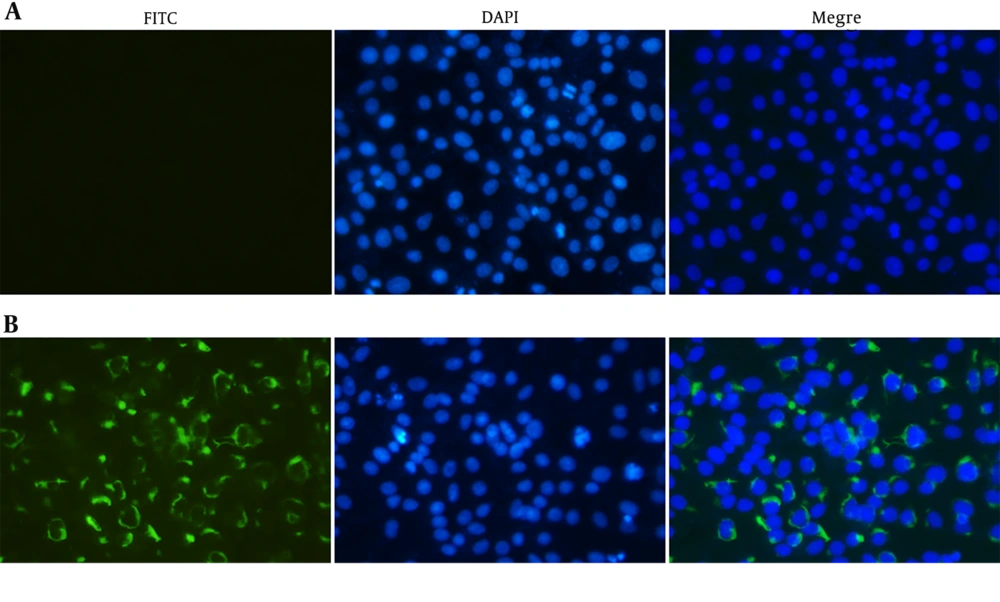
The reactivity and specificity of the polyclonal antibody against ZIKV-E was evaluated by Western Blot and immunofluorescence assays. The Figure 5 showed that anti-ZIKV-E antibody could recognize the native E protein in ZIKV infected cells, and the negative control had no fluorescence. The results of Western Blot showed that the recombinant ZIKV-E protein and native E protein in ZIKV infected cells were recognized by mouse polyclonal antisera (Figure 4, Lane 2, 3 and Figure 6, Lane 2).
Indirect immunofluorescent assay of the E protein in Vero cells infected with ZIKV. ZIKV infected (B) and mock-infected (A) Vero cells were probed with the E antiserum and observed under fluorescent light. ZIKV infected and mock-infected Vero cells were counterstained with DAPI to visualize the nuclei (40×).
4.5. Capture of ZIKV Virion in Cell Culture Medium by Polyclonal Antibody
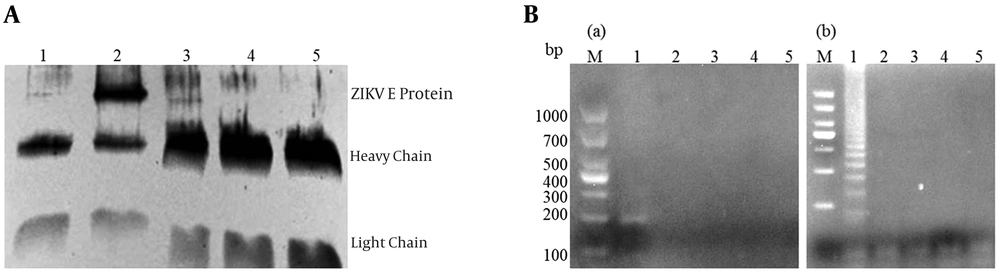
Protein A/G coated magnetic beads (Biotool) were coated with the polyclonal antibody of ZIKV-E, ZIKV-NS1, Shigella, and Salmonella to generate the immuno-magnetic beads. Then, the immuno-magnetic beads were used for capturing the supernatant of ZIKV infected, and mock-infected Vero cells. The mixtures were confirmed by Western Blot (Figure 7A). According to the manufacturer’s instructions, the mixture containing magnetic beads, antibodies, viruses were used for the extraction of cDNA. As shown in Figure 7B, a 200 bp fragment (A) and trapezoidal strip (B) were obviously observed in the polyclonal antibody of ZIKV-E and used for capturing ZIKV, however, there was no amplified in other polyclonal antibodies, which were used for capturing ZIKV.
Evaluation of the ZIKV virion capture ability of the ZIKV-E polyclonal antibody by Western Blot, IC-PCR, IC-LAMP assay. A, the supernatant of ZIKV infected Vero cells (lane 2), and mock-infected Vero cells (lane 1) were captured by the polyclonal antibody of ZIKV-E using the immuno-magnetic beads. Besides the Vero cells supernatant of ZIKV infected also captured by the polyclonal antibody of ZIKV-NS1, Shigella, and Salmonella (lane 3, 4, 5), then the mixture were used for Western Blot analysis using anti-ZIKV-E antiserum; B, (a) the IC-PCR Products were analyzed by gel electrophoresis; lane 1 positive IC-PCR products; lane 2 to lane 4 represent the cDNA were captured by the polyclonal antibody of ZIKV-NS1, Shigella, and Salmonella, lane 5 negative control; (b) The IC-LAMP Products were analyzed by gel electrophoresis; lane1, positive IC-LAMP products; lane 2 to lane 4 represent the cDNA were captured by the polyclonal antibody of ZIKV-NS1, Shigella, and Salmonella, lane 5 negative control.
5. Discussion
Much attention was focused on the ZIKV epidemic in the Americas. There is the possibility of large-scale outbreaks in the equatorial and northern hemisphere, where ~80% of the human population live (21, 22). The bacterial expression has been used widely for its rapid growth rate, relative inexpensive cost, and ease of manipulation (23). In this study, pET-32a-E prokaryotic expression vector was constructed successfully. After induction and purification, the E protein was obtained and then used as an immunogen to inoculate BALB/c mice. After immunization, the serum were collected and used for Western Blot and indirect immunofluorescence analysis. The results showed that the mouse polyclonal antibody could react with the native E protein in Vero cells infected with ZIKV specifically (24). Next, polyclonal antibody against the ZIKV-E protein was used for capturing ZIKV in the cell culture medium. In addition, the polyclonal antibody of ZIKV-NS1, Shigella, and Salmonella were used for capturing ZIKV. The Western Blot, IC-LAMP, and IC-PCR results showed that only the polyclonal antibodies of ZIKV-E have the ability to capture virion. That means the prokaryotic expression and purification of Zika virus E protein and preparation of its polyclonal antibody was able to identify some epitope on the visions. However, ZIKV-E was expressed in E. coli, which did not support the proper folding of protein; the protein refolding is required to attain its native conformation (25).
It is well known that the prokaryotic expression system lacks the functional glycosylation modification on recombinant protein, thus, the structure of recombinant E and natural E protein expression were different in a certain extent. Denaturing purification strategy was used for the formation of inclusion body, which markedly affects the conformation of the recombinant glycoprotein epitope. Various recombinant protein renature methods had been reported such as refolding in the refolding buffer (100 mM Tris-HCl, 0.5 M L L-arginine, 0.2 mM EDTA, pH 7.5) (26), using ion-exchange chromatography (27), and biochemical and biophysical methods (28). Given this reason, the protein was renatured only in PBS and then the mice were immunized. We found that the fluorescence of anti-ZIKV-E polyclonal antibody reacted with Vero cells infected with the ZIKV was stronger than the recombinant E was not renature (data not shown). The reason may be that the E protein’s structure was recovered partly after renaturation. In this experiment, the E protein was only renatured in PBS, however, the results of Western Blot and immunofluorescence assays showed that the recombinant proteins conformation was similar to its native protein in a certain extent.
5.1. Conclusions
Our findings proved that the polyclonal antibodies elicited by recombinant ZIKV-E are able to recognize native virions. In addition, it also provides the basis for further preparation of neutralizing antibodies against ZIKV and the development of the related detection kits such as IC-PCR, IC-LAMP, and sandwich ELISA. This approach could effectively reduce times for the detection of ZIKV. Furthermore, it has important implications for the multivalent vaccine development.