1. Background
Community-acquired pneumococcal infections (CAPI) are health threatening and cause morbidity and mortality in pediatric and elderly populations, but the transformation inhibition and infection control of multi-drug resistant Streptococcus pneumoniae strains remain a major health challenge (1, 2). In previous studies, the frequency of pneumococcal meningitis in elderly patients was reported more than 35% (3-5). Furthermore, emergence of S. pneumoniae strains with decreasing vancomycin susceptibility represent an important warning sign for health authorities (6). Nonetheless, the introduction of the 23-valent polysaccharide pneumococcal vaccine (PPSV23) has created hope for controlling and preventing pneumococcal infections (7).
Recent findings have corroborated the effectiveness of PPSV23 in preventing hospitalization due to S. pneumoniae community-acquired pneumonia (8). Pneumococcal conjugate vaccines (PCVs) in some countries have reduced the incidence of pneumococcal diseases associated with vaccine serotypes. Rapid increase of non-vaccine serotypes in carriage and pneumococcal infections has become a great concern for physicians (9). In addition to meningitis due to S. pneumoniae, pneumococcal infections such as surgical wound infection are reported (10). Therefore, other methods of bacterial growth inhibition are being considered. For example, the results of an investigation showed that Mucin 1 (MUC1) could protect mice against severe pneumococcal diseases, which could be mediated by enhancing macrophage phagocytosis bacterial cells (11).
A previous study showed that oral administration of Lactobacillus casei could prevent S. pneumoniae lung infection in a mouse model (12). Xylitol, which is a five-carbon sugar alcohol, has been considered as an antibacterial agent in some countries and has been used in chewing gums, lozenges, syrups, nasal sprays, toothpastes, and mouthwashes. This compound may be a promising agent in ear, nose, throat (ENT) practices, but further experimental and clinical studies are required (13). The antibacterial activities and synergistic effects of xylitol with other sugars have not been clarified yet. A research reported that xylitol had no significant impact on pneumococcal mucosal colonization (14), while in another study exposure of both epithelial cells and bacteria to 5% xylitol significantly reduced the adherence of pneumococci (15).
Former studies claimed that if xylitol reduces the growth of S. pneumoniae in the nasopharynx, it can also diminish the carriage of this pathogen and thus, have clinical significance in the prevention of pneumococcal diseases (16). Besides, another study suggested that exposure to 5% xylitol changed the ultrastructure of the pneumococcal capsule through reducing the expression of capsule gene (cpsB gene), which could explain the high clinical efficacy of xylitol in preventing otitis media (17).
Another study pinpointed that xylitol lowered LytA expression level, partly explaining the efficacy of xylitol in preventing acute otitis media (18). Nonetheless, the duration of bactericidal effect of xylitol as well as minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of this compound have not been described in detail yet. It is worth mentioning that S. pneumoniae strains colonization and their adherence to the nasopharyngeal epithelial cells are critical in triggering infectious diseases and invading other tissues (19). Therefore, any factor that can reduce bacterial populations in the nasopharynx may be able to prevent pneumococcal infections.
2. Objectives
The aim of this study was to evaluate the antibacterial effect of xylitol on bacterial growth and survival via tetrazolium salt-based colorimetric (MTT) assay.
3. Methods
3.1. Ethics Statement
This project was approved by the Ethics Committee of Baqiyatallah University of Medical Sciences (November 2, 2014, code No.: 37).
3.2. Bacterial Strains
In this investigation, five S. pneumoniae clinical isolates were studied. In a previous study, all isolated strains were identified and their serotypes were determined (6, 7). In addition, the S. pneumoniae strain ATCC 6305 (Baharafshan Laboratory, Tehran, Iran) was used as reference.
3.3. Bacterial Culture Condition
The lyophilized bacteria in aseptic conditions were dissolved in 1 mL of brain heart infusion (BHI) broth (Merck, Germany). Then, 50 μL of the suspension was transferred into a blood agar (Merck, Germany) supplemented by 5% sheep blood and incubated at 37°C in a 5% CO2 atmosphere for 24 hours. After overnight incubation, bacterial colonies were harvested and transferred into 3 mL of BHI broth containing 0.2% glucose supplemented with 10% (vol/vol) fetal bovine serum (FBS; Cambrex, Belgium). Then, the solution was incubated at 37°C in a 5% CO2 atmosphere for about 5 hours, and the OD650 was reached to 0.5 McFarland. Afterwards, bacterial suspension was adjusted to match the turbidity standard of 0.5 McFarland concentration. Then, 300 μL of this suspension was added to 3 mL of the test media that were prepared by adding sugar alcohol or various sugar concentrations. The test tubes were incubated at 37°C, and the OD570 results were calculated after 0, 3, 5, 8, 11, 15, 20, and 24 hours. Each test was carried out in triplicate.
3.4. Sugars Stock Solution Preparation and Sterilization
In this research, the 40% stock solution of each sugar was prepared separately. The tested sugars were sterilized by filtration (0.22 mm pore-sized filter; Millipore). Then, certain amount was added to related test tubes. The used media and gradients in this investigation were as follows: BHI broth + 2.5% xylitol, BHI broth + 2.5% xylitol, BHI broth + 7.5% xylitol, BHI broth + 2.5% fructose + 2.5% xylitol, BHI broth + 5% fructose + 5% xylitol, BHI broth + 7.5% fructose + 7.5% xylitol, BHI broth + 5% glucose, and BHI broth alone. Different volumes and concentrations of xylitol, fructose, and glucose were prepared from their stock solutions.
3.5. Assay of Xylitol Antibacterial Effects
The effects of different concentrations of xylitol and fructose on growth of S. pneumoniae strains were examined both separately and in combination (15). The test media were inoculated and incubated at 37°C in a 5% CO2 atmosphere for 24 hours. During an overnight (at three-hour intervals), 200 μL of each medium was transferred to a 96-well plate. Afterwards, 20 μL of MTT solution was added to each well, and the wells were incubated at 37°C in a 5% CO2 atmosphere. After 4 hours, the supernatant was discarded and 50 μL of dimethyl sulfoxide (DMSO) was added to dissolve blue colored formazan crystals. The plate was again incubated for 30 minutes, and optical density (OD) was measured with ELISA reader at the wavelength of 570 nm.
3.6. Stock Solution of MTT Preparation
A stock solution of MTT at a concentration of 5 mg/mL was prepared in phosphate-buffered saline (PBS) in pH = 7 and was kept at 4°C in the dark until used. In MTT assay, a colorimetric assay based on the ability of viable cells to reduce a soluble yellow tetrazolium salt (3-[4, 5-dimethylthiazol-2-yl] 2, 5-diphenyl tetrazolium bromide) (MTT) to blue and insoluble formazan crystals by mitochondrial succinate dehydrogenase activity of viable cells was performed with a minor modification (20). The amount of color produced is directly related to the number of cells that are metabolically active. Unlike others, in this method washing and harvesting steps, which may result in the loss of a large number of cells, were removed. All the stages of the experiment, from bacterial culture to reading the results with ELISA reader, were performed in a microplate. Therefore, the repeatability, accuracy, and sensitivity of this method are high.
4. Results
The measurement of bacterial growth revealed bacterial concentrations of 102 CFU/mL to 109 CFU/mL in various test media containing of 2.5%, 5%, and 7.5% of xylitol with or without fructose. After 24 hours of incubation, OD of the control medium containing 5% glucose was determined by UV-visible spectrophotometer. Table 1 presents means and standard deviations of bacterial growth at OD570.
| Media | 0 Hours | 3 Hours | 5 Hours | 8 Hours | 11 Hours | 15 Hours | 20 Hours | 24 Hours |
|---|---|---|---|---|---|---|---|---|
| BHI broth (control) | 0.0238 ± 0.0185 | 0.175 ± 0.0036 | 0.31 ± 0.0017 | 0.288 ± 0.0052 | 0.285 ± 0.0026 | 0.283 ± 0.0036 | 0.28 ± 0.001 | 0.17 ± 0.0036 |
| BHI broth + 5% glucose | 0.0142 ± 0.0125 | 0.165 ± 0.0026 | 0.343 ± 0.0036 | 0.315 ± 0.0036 | 0.314 ± 0.0036 | 0.31 ± 0.0036 | 0.3 ± 0.0026 | 0.151 ± 0.001 |
| BHI broth + 2.5% xylitol | 0.0175 ± 0.0131 | 0.156 ± 0.0036 | 0.191 ± 0.001 | 0.187 ± 0.0026 | 0.182 ± 0.0055 | 0.18 ± 0.0036 | 0.178 ± 0.001 | 0.157 ± 0.0026 |
| BHI broth + 5% xylitol | 0.0077 ± 0.0024 | 0.152 ± 0.0017 | 0.189 ± 0.001 | 0.177 ± 0.0052 | 0.172 ± 0.0026 | 0.171 ± 0.0036 | 0.17 ± 0.0045 | 0.138 ± 0.0043 |
| BHI broth + 7.5% xylitol | 0.0066 ± 0.0004 | 0.142 ± 0.001 | 0.186 ± 0.0043 | 0.174 ± 0.0026 | 0.171 ± 0.002 | 0.17 ± 0.0043 | 0.166 ± 0.0036 | 0.135 ± 0.0017 |
| BHI broth + 2.5% fructose + 2.5% xylitol | 0.0181 ± 0.0086 | 0.162 ± 0.0036 | 0.275 ± 0.001 | 0.265 ± 0.00608 | 0.261 ± 0.0017 | 0.26 ± 0.0036 | 0.258 ± 0.003 | 0.146 ± 0.0026 |
| BHI broth + 5% fructose + 5% xylitol | 0.0059 ± 0.00308 | 0.163 ± 0.0017 | 0.286 ± 0.001 | 0.276 ± 0.0026 | 0.266 ± 0.0026 | 0.264 ± 0.0043 | 0.262 ± 0.0017 | 0.13 ± 0.0075 |
| BHI broth + 7.5% fructose + 7.5% xylitol | 0.0429 ± 0.0201 | 0.165 ± 0.001 | 0.299 ± 0.0026 | 0.284 ± 0.0036 | 0.277 ± 0.0017 | 0.275 ± 0.0036 | 0.262 ± 0.0055 | 0.123 ± 0.0026 |
Abbreviation: BHI, brain heart infusion.
a Values are expressed as mean ± SD.
4.1. Xylitol Antibacterial Effects
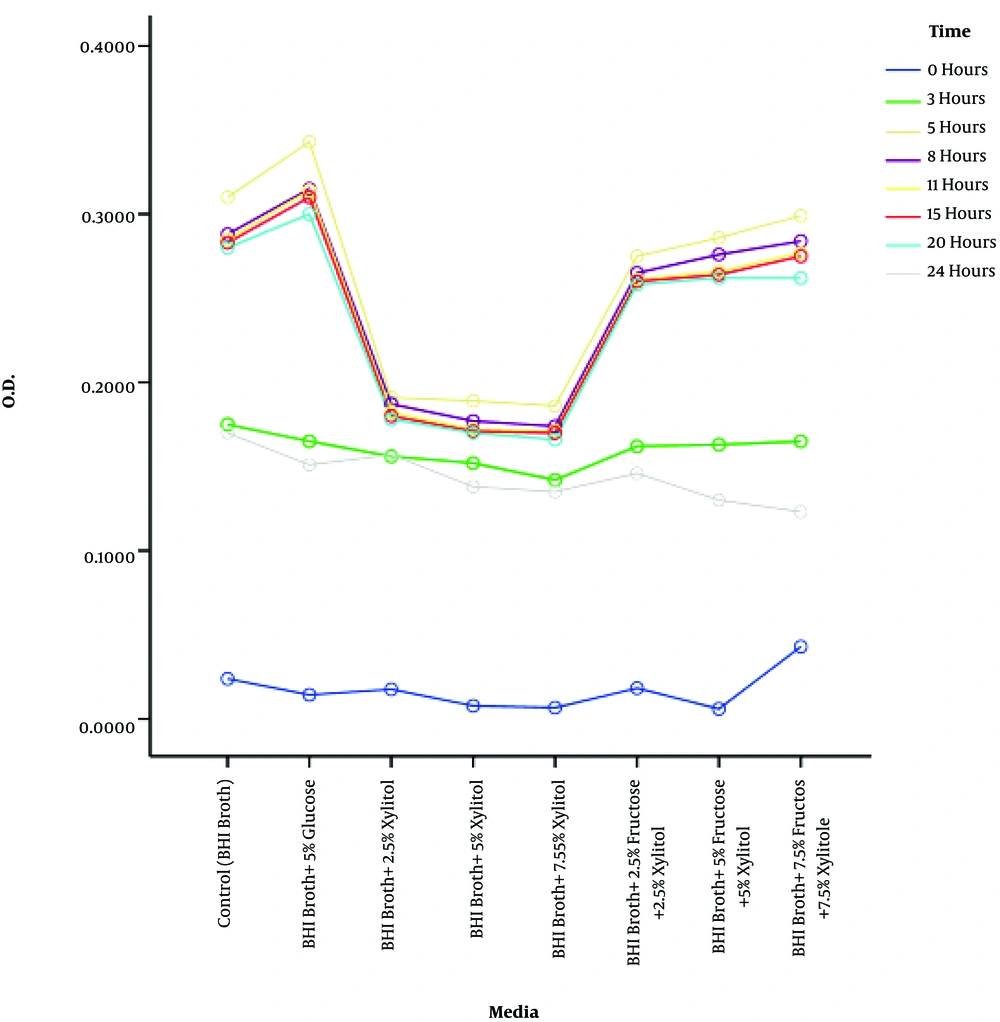
In order for accurate evaluation of the inhibitory effect of xylitol on the growth of S. pneumoniae strains, reaction conditions were normalized and same conditions were prepared for all the strains examined. To achieve this, the suspensions of growing bacteria were provided in a logarithmic phase. According to spectrophotometric results, the same volumes of suspensions at an OD of 0.3 - 0.4 (about 0.5 McFarland concentration) were transferred from each strain to various test tubes containing 7.5%, 5%, and 2.5% of xylitol in BHI broth. Results regarding the effect of each of the above-mentioned xylitol concentrations at different times in 24 hours period are illustrated in Figure 1.
Kruskal-Wallis nonparametric test indicated that the 2.5%, 5%, and 7.5% concentrations of xylitol were associated with a reduction in bacterial growth, and the effects of different concentrations in various environments and times were statistically significant (P < 0.0001).
Furthermore, Bonferroni’s post hoc test showed a significant growth difference in various test media containing 2.5%, 5%, and 7.5% concentrations of xylitol. According to the results of pairwise comparisons, the effects of various media, especially the control medium, compared with the media containing the above-mentioned concentrations of xylitol were significantly different (P < 0.001). In addition, counting the number of bacteria by MTT assay indicated that the growth rate of bacteria varied in different environments, but it followed the same trend. However, the simplicity and efficacy of the MTT assay were suitable for determining the anti-bacterial effect of xylitol. In other words, MTT assay is more precise in viable cell count compared to the serial dilution method and direct counting of bacterial colonies, and it stands in correlative relation to direct OD calculation method at a wavelength of 570 nm.
The results of this study revealed that the least mean of OD, in other words, the lowest number of bacterial cells, was related to the media containing 2.5%, 5%, and 7.5% concentrations of xylitol, respectively. Also, mean OD decreased during time, which indicates the inhibitory effect of xylitol on bacterial growth that was proven in the previous research as well. One of the important findings of this study was that in the presence of fructose, xylitol antibacterial effects declined.
As it was noted in this study, the media containing xylitol and fructose showed a significant difference in the mean OD relative to the medium treated with xylitol alone. However, the highest bacterial growth rate was related to the medium containing 5% glucose. Comparison of bacterial growth between the medium containing 5% glucose and the control medium showed that bacterial growth rate was lower in the medium without 5% glucose.
5. Discussion
In this study, the growth inhibitory effect of xylitol on S. pneumoniae strains was examined by different methods including the microdilution method (21), with minor modified MTT method with tetrazolium violet as a growth indicator (22), and gene expression inhibition method (data not shown). The results revealed that adding different concentrations of xylitol, fructose, and glucose to each well containing 200 µl of enriched BHI broth and 2.5 × 103 CFU/well caused no significant variation in OD absorption, which was not considered. In the medium containing 5% glucose, after 8 hours of incubation the maximum growth was obtained (109 CFU/well at an OD of 3 nm) and stagnated for up to 24 hours. However, bacterial growth rate in the media containing 2.5%, 5%, and 7.5% of xylitol was reduced significantly after 3 hours of incubation in comparison with the control medium. The combination of various concentrations of xylitol with 5% fructose inhibited the antibacterial effects of xylitol (Figure 1). These findings support the fact that xylitol alone shows antibacterial activity, but in combination with fructose its antibacterial effect is eliminated. The reason for this finding is unclear. This suggests that using fructose as a preservative or stabilizing agent for xylitol is not appropriate.
Life-threatening infections caused by S. pneumoniae still remain a global health problem, especially in children, the population above 50 years of age, and immunocompromised patients. Thus, controlling and preventing pneumococcal infections in different groups is one the most important health priorities. In this regard, the administration of a dose of 13-valent pneumococcal conjugate vaccine first and then 23-valent polysaccharide pneumococcal vaccine (PPV23) at least two months later has been recommended (23). PPV23 protects elderly patients from hospitalization due to SpCAP, but female sex drives the effectiveness showed needed future analysis of vaccine trials should necessitate (8). Nonetheless, the diversity of S. pneumoniae capsular polysaccharides results in more than 91 serotypes, of which at least 23 are virulent in vaccines (24). Despite the existing effective vaccines, they cannot cover all S. pneumoniae pathogenic strains. Furthermore, emergence of multi-drug resistant S. pneumoniae causing community-acquired pneumonia has added to the problems (25). In this regard, finding a non-related antibiotic therapy may prove useful for controlling S. pneumoniae infections worldwide.
5.1. Conclusions
Based on our findings, xylitol alone at 5% to 7.5% concentrations showed antibacterial activity against S. pneumoniae, and it killed about 80% to 100% of cells under laboratory conditions after 3 to 7 hours, respectively. Thus, the use of xylitol as a food additive may be effective in controlling pneumococcal superficial or gastrointestinal tract infections. However, based on our study, it must be noted that when mixed with fructose, antibacterial effect of xylitol is decreased.