1. Background
Nontypeable Haemophilus influenzae (NTHi) is one of the major causative pathogens responsible for acute otitis media (1, 2). The conventional antimicrobial treatments of acute otitis media lead to a significant risk of recurrence of acute otitis media caused by NTHi, presumably as a result of biofilm formation (3, 4). A biofilm is a dynamic condition consisting of bacterial microcolonies enclosed within a highly hydrated self-produced extracellular matrix. Biofilms can be evaluated by a variety of research techniques. It is well known that communal bacteria that form biofilms are highly resistant to the bactericidal effects of antibiotics than are free-living, planktonic bacteria (5-9). According to various reports, bacteria such as Pseudomonas aeruginosa and fungi such as Candida albicans grow in biofilms, and cells in such biofilms differ from their planktonic counterparts (10-13). An in vitro co-culture model showed NTHi cells adhered to and formed antibiotic-resistant biofilms on the airway epithelium of humans (14).
The recognition of the roles that NTHi biofilm plays in acute otitis media has improved our understanding of the reasons why persistent acute otitis media infections occur (7, 8, 15). Haemophilus influenzae often persists and establishes communities in biofilms that provide resistance to both host clearance and the bactericidal activity of antibiotics (16-19). Our previous study highlighted the fact that even though NTHi is susceptible to antimicrobial treatment, its biofilms formation is a major cause of acute otitis media cases that do not improve by treatment with AMPC (20).
On the other hand, there is little clinical guidance delineating treatments that would successfully clear NTHi biofilms. Previous studies have not provided much guidance for successful treatments although they imply the importance of biofilms as viable treatment targets (21-23). Studies have discussed methods of clearing biofilms with a single antibiotic or with concurrent or combined antibiotics (23-25). The first-line therapy for acute otitis media has been treatment with AMPC or clavulanic acid/amoxicillin (CVA/AMPC) (26-29). However, high tissue concentrations are necessary to clear bacteria in biofilms. Nonetheless, there is still no consensus as to the effective treatment for diseases involving biofilm formation (30).
Tosufloxacin is a quinolone antibiotic that has been approved for pediatric acute otitis media in Japan since 2010. Tosufloxacin exhibits potent activity against clinical isolates of H. influenzae from otological and other respiratory tract infections with favorable pharmacokinetics after oral administration to humans (31).
2. Objectives
The present study investigated the in vitro antibacterial activity of tosufloxacin against a NTHi strain isolated from pediatric acute otitis media patients.
3. Methods
3.1. Bacterial Strain and Growth Conditions
This study used an otopathogenic clinical strain of NTHi IH-202 isolated from the middle ear fluid of intractable pediatric cases of acute otitis media. As a result of examinations of the capsular type using H. influenzae antiserum (Denka Seiken, Japan), the clinical isolate was confirmed to be a non-encapsulated strain. The strain was reconstituted from a frozen glycerol stock and cultured on chocolate agar plates (Nippon Becton Dickinson Company Ltd., Japan) overnight at 37°C under a 5% CO2 environment. Then, the strain was cultured in brain heart infusion (BHI) broth (Difco Laboratories, USA) supplemented with 15 µg/mL of hemin (Sigma, USA) and 15 µg/mL of NAD (Sigma, USA) until mid-log phase (OD660nm = 0.5) at 37°C under a 5% CO2 environment. The strains used for these experiments were within the third generation to prevent them from losing their phenotypic characteristics. Based on the result of the crystal violet assay for the biofilm formation ability described previously (25), this strain showed a high ability to form biofilm when compared with NTHi strains from a similar origin.
3.2. Antimicrobial Agents
Tosufloxacin tosilate synthesized at Toyama Chemical (Tokyo, Japan) was provided by Taisho Toyama Pharmaceutical Co., Ltd. The in vitro pharmacokinetic parameters of tosufloxacin were 0.60 µg/mL of Cmax (peak serum concentration), 3.59 hours of t1/2, and 10.5 µg/mL.h of AUC0-24h (area under the curve) at a clinical oral dosing of tosufloxacin tosilate at 150 mg t.i.d. Those parameters at 300 mg b.i.d. were 1.20 µg/mL of Cmax, 3.59 hours of t1/2 and 10.5 µg/mL.h of AUC0-24h (31, 32). Cefditoren pivoxil (CDTR-PI) from WAKO Chemical Co., Japan, was used in this study. The in vitro pharmacokinetic parameters of CDTR were 1199.2 µg/mL of Cmax, 1.33 hours of t1/2 and 3145.4 µg/mL.h of AUC0-7h at a clinical oral dosing of CDTR-PI at 100 mg. The concentrations of both drugs were shown as their activated forms in this experiment.
3.3. Antimicrobial Susceptibility
The minimal inhibitory concentration (MIC) was determined according to the broth dilution method recommended by the Clinical and Laboratory Standard Institute (CLSI) (M100-S21; 2011) (33). The medium used for MIC determination was cation-adjusted Mueller-Hinton broth (Becton-Dickinson, Japan) including 5 mg/mL yeast extract (Becton-Dickinson), 15 µg/mL hemin, and 15 µg/mL ß-NAD (Sigma-Aldrich, Japan). The bacterial suspension was inoculated onto 96-well microplates (Nunc; Kracker Scientific, USA) containing the drug solution (104 colony-forming units [CFU]/well). The MICs were determined after incubation at 35°C for 21 hours.
3.4. Biofilm Assay
Biofilm formation by the NTHi IH-202 strain was evaluated by the bacteria and epithelial cell co-culture model. Briefly, human pharyngeal epithelial cells, Detroit 562 cells, were cultured in a 27-mm glass base dish (AGC TECHNO GLASS CO., Japan) using Eagle’s Minimum Essential Medium (MEM) (Gibco®, Thermo Fisher Scientific, Japan) containing 10% fetal bovine serum (FBS) at 37°C under a 5% CO2 environment until a confluent growth occurred. Then, the NTHi IH-202 strain was added to the Detroit 562 cell culture dish at 1×106 CFUs/mL and incubated for 6 hours at 37°C under a 5% CO2 environment. After removing planktonic NTHi by washing with fresh sterile MEM, the NTHi cells adhering to the Detroit 562 cells were prepared for biofilm evaluation. The NTHi IH-202 strain cells adhering to the Detroit 562 cells was cultured to form biofilm at 37°C under a 5% CO2 environment with perfusion of MEM containing 1% FBS at 0.3 mL/min by a peristatic pump. After 20 hours of culture, the presence of NTHi IH-202 strain cells in the biofilm was confirmed and the film was exposed to tosufloxacin or CDTR.
3.5. Visualization of NTHi Biofilm
The NTHi biofilm was fixed in 4% paraformaldehyde. After washing three times with Ringer's solution, the NTHi biofilm was incubated with biotin-conjugated pure Limulus polyphemus lectin (LPA) from horseshoe crabs (EY Laboratories, USA) with a 1:100 dilution ratio with Ringer's solution at 37°C for 60 min. Then, the NTHi biofilm was incubated with ABC reagent (Vector Laboratories, USA) diluted at 1:100 with Ringer's solution for 30 min at room temperature. After washing three times with Ringer's solution, the NTHi biofilm was incubated with streptavidin Alexa Fluor® 405 conjugate (Molecular Probes, Japan) diluted 1:500 with Ringer's solution for 30 min at room temperature. Finally, NTHi biofilm was enclosed with ProLong Gold Antifade Reagent (Molecular Probes, Inc.) and imaged using LSM-700 laser scanning confocal microscopy (LSCM).
3.6. Visualization of Live/Dead NTHi in Biofilm
The viability of NTHi cells in biofilm was visualized as follows. The NTHi cells in the biofilm were incubated with LIVE/DEAD® BacLight™ Bacterial Viability kit (Molecular Probes, Inc., Thermo Fisher Scientific K.K.) for 15 min at room temperature after washing three times with Ringer's solution. The bacterial viability was visualized using LSM-700 laser scanning confocal microscopy (LSCM) after washing three times with Ringer's solution. Image processing of the 3-D image of the confocal microscopy and counts of live or dead cells were performed using IMARIS (Bitplane, Switzerland).
3.7. Numbers of Live NTHi Cells in Biofilm
After the antimicrobial exposures, the NTHi cells forming the biofilm on the Detroit 562 cells were disrupted with 0.025% Triton X and cultured on BHI agar (Nippon Becton Dickinson Company Ltd.) containing 15 µg/mL hemin and 15 µg/mL β-NAD for 24 hours under a 5% CO2 environment. The number of NTHi cells (CFUs) in the biofilm was counted to determine the number of live NTHi cells after antimicrobial exposures.
3.8. Statistical Analysis
The statistical comparisons of the numbers of CFUs of NTHi after antimicrobial exposure were performed using the One-way ANOVA test with Dunnett test. The statistical comparisons of the ratio of live or dead NTHi cells after antimicrobial exposure were performed using the chi square test. All the statistical analyses were performed using Prism 5 (GraphPad Software, Inc., USA). P-value less than 0.05 was considered statistically significant.
4. Results
4.1. Antimicrobial Susceptibilities
The MICs of tosufloxacin and CDTR-PI to the NTHi IH-202 strain were 0.004 µg/mL and 0.125 µg/mL, respectively.
4.2. Development of NTHi Biofilm
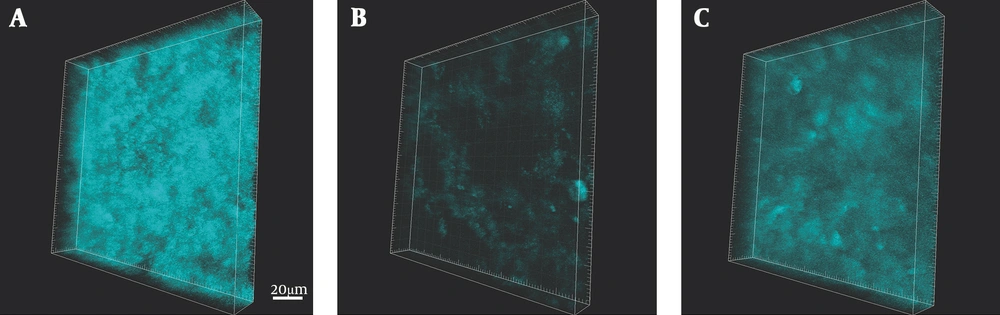
We visualized the amount of NTHi biofilm formation by staining with LPA after 20 hours of antimicrobial exposure (Figure 1). In the control without antibiotics, the NTHi formed a dense biofilm (Figure 1A). In contrast, exposure of the biofilm to tosufloxacin at 0.96 µg/mL remarkably reduced the amount of NTHi biofilm formed (Figure 1B). The reduction of biofilm formation after exposure to CDTR at 1.43 µg/mL was weaker than the reduction that occurred when the NTHI biofilm was exposed to tosufloxacin (Figure 1C).
NTHi biofilm on Detroit 562 cell visualized by Limulus polyphemus lectin (LPA) stain. NTHi biofilm after 20 hours of exposure to either tosufloxacin (0.96 µg/mL) or CDTR (1.43 µg/mL) was fluorescently stained with biotin-conjugated pure Limulus polyphemus lectin (LPA) from horseshoe crabs. A, control (without antibiotics); B, Tosufloxacin 0.96 µg/mL (x256 MIC); C, CDTR 1.43 µg/mL (x190 MIC). Scale bar = 20 µm.
4.3. Evaluation of Numbers of Alive NTHi Cells in Biofilm
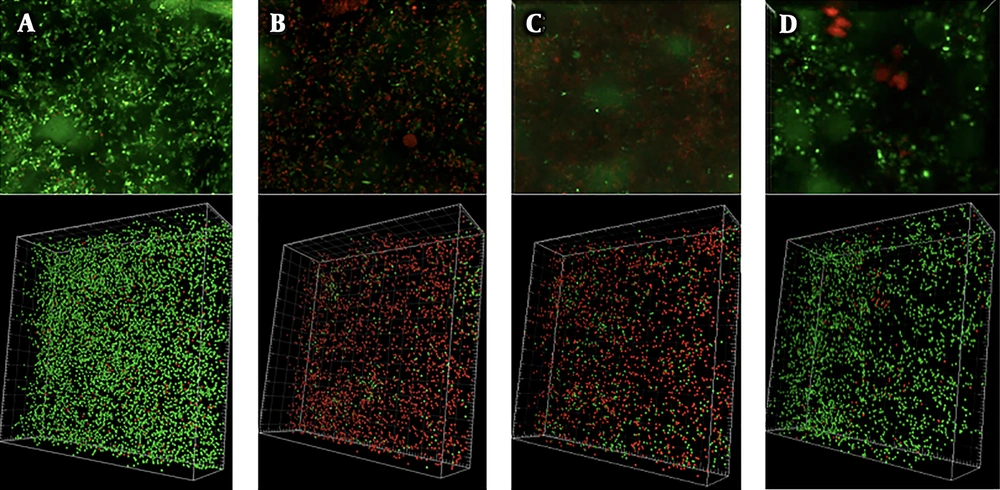
After 20 hours of antimicrobial exposure, we evaluated the viabilities of the NTHi cells in the biofilm using LIVE/DEAD staining. In the control, most of the NTHi cells were alive (Figure 2A). On the other hand, incubation with tosufloxacin at both 0.96 µg/mL and 0.74 µg/mL killed most of the NTHi cells in the biofilm. In this experiment, we evaluated the bactericidal effect of tosufloxacin at both Cmax and x190 MIC (this ratio is similar to the ratio used for CDTR). We found that 70% and 84% of NTHi cells were killed by tosufloxacin at 0.96 µg/mL and 0.74 µg/mL, respectively (Figures 2B, C and Table 1). However, the bactericidal effect of CDTR at 0.143 µg/mL was weaker than the results from the two concentrations of tosufloxacin and killed only 6.7% of NTHi cells (Figure 2D and Table 1).
Bactericidal effects of antibiotics on NTHi cells in biofilm. The amounts of viable NTHi cells in biofilm after 20 hours of exposure to either tosufloxacin (0.96 µg/mL and 0.72 µg/mL) or CDTR (1.43 µg/mL) were visualized using the LIVE/DEAD® BacLight™ Bacterial Viability kit fluorescence stain. Green: alive NTHi cells, Red: dead NTHi cells. Control was NTHi in biofilm without antibiotics. A, control (without antibiotics); B, tosufloxacin 0.96 µg/mL (x256 MIC); C, tosufloxacin 0.72 µg/mL (x190 MIC), CDTR 1.43 µg/mL (x190 MIC).
| CDTR, 1.43 μg/mL (x190 MIC) | Tosufloxacin, 0.72 μg/mL (x190 MIC) | Tosufloxacin, 0.96 μg/mL (x256 MIC) | |
|---|---|---|---|
| Live NTHi cells, % | 93.3 | 30.0 | 16.0 |
| Dead NTHi cells, % | 6.7 | 70.0a | 84.0 |
a P < 0.01 by chi square test.
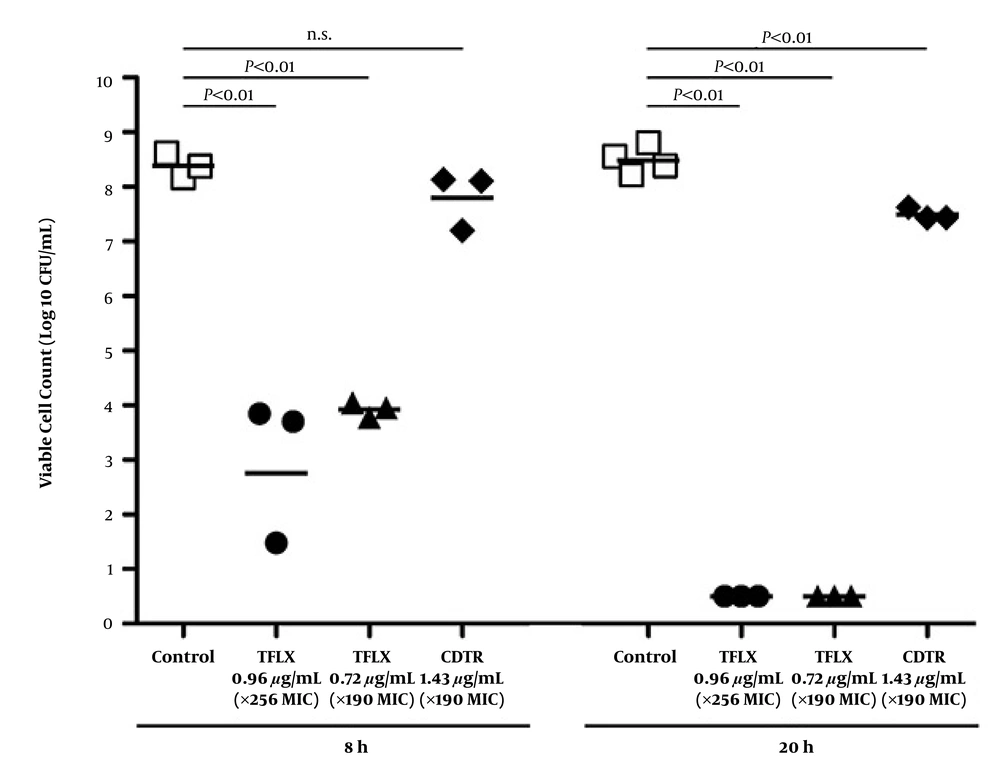
We further evaluated the live NTHi cells by culture after antimicrobial exposures for 8 hours to 20 hours (Figure 3 and Table 2). The numbers of live NTHi cells at 8 hours and 20 hours in the controls were 8.39 Log10 CFU/mL and 8.49 Log10 CFU/mL, respectively. Incubation with x256 MIC of tosufloxacin significantly reduced the number of live cells in the biofilm to 3.01 Log10 CFU/mL after 8 hours and killed all the NTHi cells after 20 hours (P ≤ 0.01, P ≤ 0.01). Incubation with x190 MIC of tosufloxacin also reduced the number of live cells in the biofilm to 3.93 Log10 CFU/mL after 8 hours and killed all the NTHi cells after 20 hours (P ≤ 0.01, P ≤ 0.01). On the other hand, incubation with x190 MIC of CDTR for 8 hours failed to reduce the number of live NTHi cells in the biofilm, resulting in 7.81 Log10 CFU/mL. Incubation with x190 MIC of CDTR for 20 hours resulted in a relatively reduced number of live NTHi cells (7.50 Log10 CFU/mL).
a P < 0.01 by ANOVA test.
5. Discussion
NTHi biofilm has become of broad interest when evaluating effective antimicrobial treatments against intractable acute otitis media. The current study indicated the bactericidal efficacies of tosufloxacin against NTHi cells in biofilm. These findings suggest an alternative antimicrobial treatment strategy against intractable cases of acute otitis media caused by NTHi biofilms. The observations in human and experimental animal studies in vivo have demonstrated that biofilm is involved in the intractable pathogenesis of acute otitis media (34-38). Post revealed that biofilm is formed on ventilation tubes removed from children with refractory acute otitis media (36). The numbers of bacteria in biofilms on and within the epithelial cell surfaces of adenoids evaluated by 16S rRNA-based CLSM and FISH were higher in children with chronic otitis media than in children with sleep apnea syndrome (34, 37, 38). Even though AMPC has long been recommended as the first drug of choice for acute otitis media, a recent study has further revealed that sub-inhibitory concentrations of β-lactams promoted biofilm formation with increasing the expression of glycogen-production-related genes (39).
While biofilm has previously been evaluated using static biological materials such as crystal violet stain in vitro (40, 41), we applied an NTHi and epithelial cells co-culture model to represent in vivo NTHi biofilm formation. This system is better at representing in vivo biofilm changes. Bacterial biofilms grown on Detroit 562 eukaryotic cells could be differentiated visually by the use of the confocal laser scanning microscope with Live/Dead DNA stain, while syto9 and propidium iodide stain eukaryotic and prokaryotic DNA and LPA bind to human cells. The sugar-enriched extracellular matrix of biofilm protects bacteria from phagocytic killing and from the bactericidal effect of antibiotics (42-44). Bacteria in biofilm exhibit different growth rates than free living, planktonic bacteria during the rapid growth periods to a dormant or sessile state (45). The clinical NTHi isolates expressing phosphorylated choline exhibited stable maturation of biofilm and inhibited early inflammation (46, 47). Our results, which were obtained through different experimental methods than those used in previous studies, demonstrate the potent activity of fluoroquinolones against NTHi biofilms even though planktonic cells would be unaffected by the same treatment.
A clinically attainable concentration of tosufloxacin showed biofilm destruction and bactericidal activity against biofilm-forming NTHi in shorter exposure periods such as 8 hours. On the other hand, CDTR required high concentrations and relatively longer exposure periods such as 20 hours to show a bactericidal effect against NTHi cells in biofilm. However, CDTR’s bactericidal effect was weaker than the effect afforded by tosufloxacin. The difference between the bactericidal effects of tosufloxacin and CDTR may depend upon whether the effects of these two bactericidal agents are dose-dependent or time-dependent according to PK/PD logic (21, 48). In this study, the concentrations of tosufloxacin and CDTR exposed to NTHi were determined based on the estimated Cmax concentrations of the middle ear tissue of healthy children. In addition, tosufloxacin resulted in a significant reduction in the amount of NTHi biofilm and thus may do a good job of penetrating established NTHi biofilms. Fluoroquinolone showed bactericidal activity on biofilm-forming bacteria, with an image showing that the matrix had been destroyed. This result was considered to occur because fluoroquinolone penetrates exopolysaccharide and shows bactericidal activity against biofilm-forming bacteria in various growth states, including against bacteria in the sessile state (21).
Sugiura et al. reported that tosufloxacin has potent antibacterial effects against NTHi including β-lactam-resistant strains isolated from pediatric acute otitis media (32). Tosufloxacin reduced inviable cell counts from the initial inoculum after exposure to 100 times the MIC, which is equivalent to the free-drug AUC0-24 (fAUC0-24) at a clinical dosage in pediatric patients for 24 hours or 10 times the MIC (1/10 of fAUC0-24). As for a quinolone-susceptible S. pneumoniae strain, tosufloxacin showed bactericidal activity, given that both the AUC0-24h/MIC ratios at the dosages of 150 mg t.i.d. and 300 mg b.i.d. of tosufloxacin tosilate were 138 and 193, and the Cmax/MIC ranges were 7.93 - 10.2 and 15.9 - 17.6, respectively, which were greater than those of levofloxacin (100 mg t.i.d. and 200 mg b.i.d.). The greater area above the killing curves and the shorter time to achieve 99.9% killing in both models of tosufloxacin when compared with those of LVFX were related to their larger AUC0-24h/MIC and Cmax/MIC. In these comparative experiments, we demonstrated that tosufloxacin had comparable or better bactericidal activity than LVFX.
A limitation in the current study is that its focus is narrow, being limited to only two antibiotics and a single NTHi strain. To properly demonstrate the significance of our findings to the larger scientific community and to confirm the broad applicability of the results presented here, the next step would be to expand our study by evaluating multiple antibiotics and several NTHi strains. However, our current results suggest the efficacy of TLFX against bacterial biofilms. Our previous on a similar subject (25) demonstrated the minimal biofilm eradication concentrations of quinolone.
5.1. Conclusions
Tosufloxacin will be an effective alternative for the treatment of acute otitis media cases caused by NTHi biofilm and has an advantage over CDTR for the treatment of intractable cases of acute otitis media involving NTHi biofilms. Our in vitro bacteria- epithelial cells co-culture model is a relatively simple and static one when compared with some other reported methods of studying NTHi biofilms. Quinolones such as tosufloxacin show potent bactericidal activity against biofilm-forming NTHi at the usual clinical dosage and are considered valuable anti-bacterial agents for the treatment of infectious diseases caused by NTHi, even when the bacteria are in the biofilm state.