1. Background
Nosocomial infections or hospital-acquired infections(HAI), are a major public health problem in hospitals worldwide, accompanied by high rate of morbidities and mortality among hospitalized patients (1). The burden of HAI is already substantial in developed countries, where it affects from 5% to 15% of hospitalized patients in regular wards and as many as 50% or more of patients in intensive care units (ICUs).In developing countries, the magnitude of the problem has remained underestimated or even unknown mostly because HAI diagnosis is complex and surveillance activities to guide interventions require expertise and enough resources (2).
The most frequent nosocomial infections are infections of surgical wounds, urinary tract infections and lower respiratory tract infections. According to WHO study, the highest prevalence of nosocomial infections occurs in intensive care units and in acute surgical and orthopaedic wards. Infection rates are higher among patients with increased susceptibility because of old age, underlying diseases, or chemotherapy (3).
Invasive medical procedures in the intensive care units remarkably increase the risk of such infections. Ventilator associated pneumonia and catheter associated urinary tract infections have been shown to cause the greatest risk to patients safety. Moreover, the use of different kinds of catheters, endotracheal tubes, supplying apparatuses and surgeries are the most common ways for transmission of nosocomial infections (4, 5). Colonization of the respiratory tract is very common in intubated patients requiring intensive care and in most instances leads to the increase of infection (1, 6). Intubation with mechanical ventilation increases the risk of pneumonia 6 to 20 folds more among patients and is associated with crude mortality rates of 20% to 40% (7, 8).
Tracheal colonization by significant number of potential pathogenic bacteria predispose patients to super infection with presentation of fever, lower respiratory signs and symptoms, and an increase in the number and proportion of polymorphonuclear leukocytes in the sputum (9). In consequence, nosocomial pneumonia is a common and a life threatening problem among seriously ill patients who are mechanically ventilated. The incidence varies from 9% to 68% with a high fatality rate ranging from 50% to 80%, especially when it is caused by antibiotic-resistant bacteria. This emergence of antibiotic-resistant microorganisms in critically ill patients represents a new challenge for intensive care physicians (10). Bacterial infection due to Gram negative bacilli in the lower respiratory tracts remains a main complication of tracheal intubation in patients requiring ventilator equipments (6).Widespread use of antibiotics in intensive care units is a potential cause of the emergence of nosocomial infections caused by antibiotic-resistant Gram-negative bacteria(11).
The percentages of Healthcare Associated Infection (HAIs) and bacterial resistance in developing countries are 3 to 5 times higher than international standards. HAIs increase the length of stay (10 days), costs (US $5000 to US $12,000), and mortality by 2 to 3folds (12).
2. Objectives
The aim of present study was to determine the presence or absence of bacterial infections in tracheal tubes and determination of their antimicrobial susceptibility patterns.
3. Materials and Methods
This cross sectional study was performed from March 2009 to March 2010 in Golestan teaching hospital in Ahvaz, south west of Iran. Specimens were collected from tracheal tubes of patients with endotracheal aspiration, who had clinical manifestations of pneumonia and were admitted to different wards of general Intensive Care Unit (ICU), Neurologic Intensive Care Unit (NICU), Neurology, pediatrics ICU, Neurosurgical ICU and transplant wards. The length of stay of patients in mentioned wards was at least two weeks prior to sampling. Tracheal tube samples were inserted in a sterile tube and transferred to the laboratory where inoculated into thioglycollate broth and incubated for 24 hours in 37oC. The broth was primarily examined for the presence of grown bacteria by a direct Gram-stained smear in the subsequent day. subculture was made for positive samples on chocolate agar, McConky agar, and blood agar and incubated in 37°C for 24 to 48 hours. then, the macroscopic and microscopic features was studied (study of shape and color of colonies and Gram staining) and based on their morphology, standard identification biochemical tests for Gram negatives including oxidase and catalase, reaction in triple sugar iron agar (TSI) medium, indole production and motility, urea utilization and catalase, coagulase and DNase tests for presence of staphylococci were performed (13).
Antimicrobial susceptibility testing was performed afterward in order to isolate bacteria by disk diffusion method as per Clinical and Laboratory Standards Institute (CLSI) standard guideline (14).The antibiotic discs which were used for Gram negative bacilli were: gentamicin (10μg), cefixime (5μg), ceftriaxone (30μg), ciprofloxacin (5μg),nalidixicacid (30μg), co-thrimoxazol (1.25/23.75μg), nitrofurantoin (300μg), tetracycline (30μg), pipracillin (100μg), imipenem (10μg), and for Gram positive cocci were: cephalothin (30μg), cefixime (5μg), ceftriaxone (30μg), ciprofloxacin (5μg), clindamycin (2μg), co-thrimoxazol (1.25/23.75μg), nitrofurantoin (300μg), oxacillin (1μg), rifampin (5μg), vancomycin (30μg). The data were analyzed by descriptive statistics using SPSS version 17.
4. Results
From total investigated specimens, 278 had positive culture with 508 isolates. The positive specimens were belonged to 191 male and 87 female hospitalized patients. The majority of these specimens (40.3%), were isolated from patients in age group of 18 to 40 years. Most of the samples were collected from intubated patients in ICU (n =. 112), and NICU (n= 107). Based on the bacteriology results, Enterobacter spp. with 209 cases (41.14%) were the most prevalent genera isolated from positive cultures. The number and frequency of other isolated bacteria were: P. aeruginosa78 (15.35%), E. coli71 (13.97.2%), coagulase negative staphylococci 75 (14.76%), S. aureus71 (13.97%), and proteus spp. 4 (0.79%).
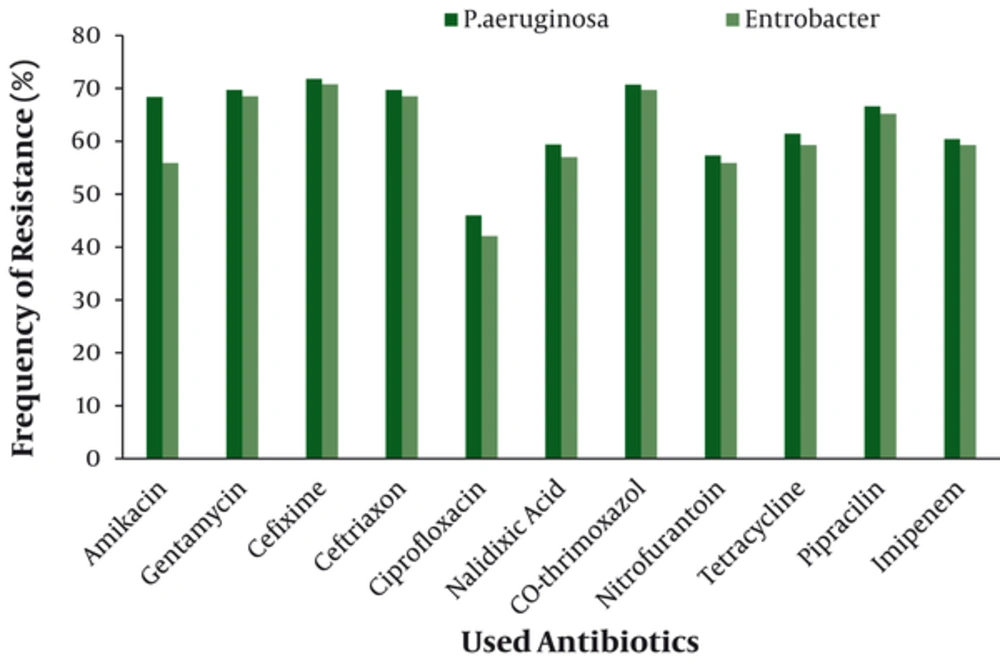
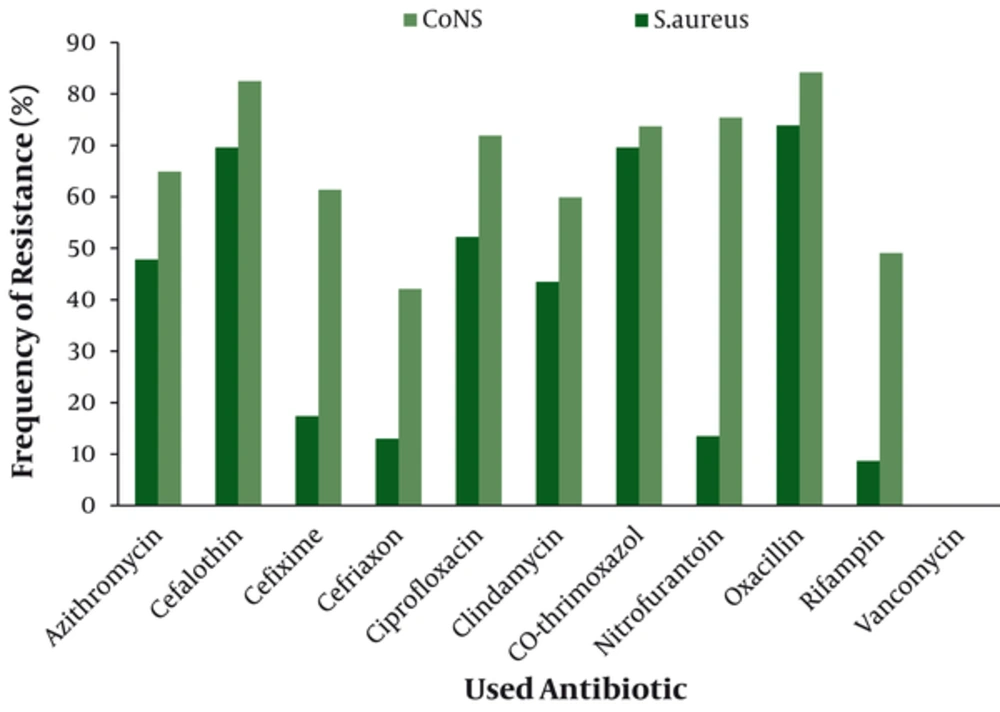
Antimicrobial susceptibility testing revealed that the most resistant Gram negative isolate was P.aeruginosa with highest resistance against cefixime (70.8%) (Figure 1). Coagulase negative staphylococci (CoNS) was the most resistant Gram positive isolate with highest resistance against oxacillin (84.2%) (Figure 2).The overall results of susceptibility testing are shown in Table 1.
| Antibiotic | P.aeruginosa | E.coli | Entrobacter | Antibiotic | S.aureus | CoNS |
|---|---|---|---|---|---|---|
| Amikacin | 68.4 | 62.4 | 55.9 | Azithromycin | 47.8 | 64.9 |
| Gentamycin | 69.7 | 59.1 | 68.5 | Cefalothin | 69.6 | 82.5 |
| Cefixime | 71.8 | 42.7 | 70.8 | Cefixime | 17.4 | 61.4 |
| Ceftriaxon | 69.7 | 52.3 | 68.5 | Cefriaxon | 13 | 42.1 |
| Ciprofloxacin | 46 | 52.7 | 42.1 | Ciprofloxacin | 52.2 | 71.9 |
| Nalidixic Acid | 59.4 | 67.3 | 57 | Clindamycin | 43.5 | 59.6 |
| Co-thrimoxazol | 70.7 | 64.5 | 69.7 | Co-thrimoxazol | 69.6 | 73.7 |
| Nitrofurantoin | 57.3 | 53.6 | 55.9 | Nitrofurantoin | 13.5 | 75.4 |
| Tetracycline | 61.4 | 60.7 | 59.3 | Oxacillin | 73.9 | 84.2 |
| Pipracilin | 66.6 | 55.5 | 65.2 | Rifampin | 8.7 | 49.1 |
| Imipenem | 60.4 | 44.5 | 59.3 | Vancomycin | - | - |
Abbreviations: CoNS,Coagulae negative staphylococci
5. Discussion
Nosocomial infections are a serious concern in teaching hospitals in Ahvaz, Iran as many other parts of the world. Despite the careful managements of the wards by the infection control committee, we have still witnessed such infections due to several factors involved in emerging of infections, for instance employing intensive medical procedures especially for immune-compromised patients. In this study which was performed in some wards of the hospital, Enterobacter spp. and P. aeruginosa were the most prevalent bacteria isolated from tracheal tubes of the patients. In the study of Andair et al., Enterobacter spp., P. aeruginosa, and S.aureus were mostly isolated (15), which was in concordance with the present study, except for CoNS which was the most common Gram positive contaminant of tracheal tubes. However the study of Amini et al. was against the findings of the present study, which in overall they reported S. aureus as the most common isolate and enterobacter as the least one, isolated from tracheal tubes in Tehran (4).
In a similar study undertaken by Rahbar and Hajia in 2006, Gram negative bacteria were accounted for 75% of total positive cultures with Klebsiella pneumonia (20%) and S. aureus (15.2%) as the most prevalent Gram negative and Antibiotic P.aeruginosa E.coli Entrobacter Antibiotic S.aureus CoNS Gram positive isolates respectively (16). While the frequency of enterobacter was 41.14% in this study, they have reported the frequency of 3% for the same bacterium, although the frequency of S. aureus in their study with 15.2% was slightly higher but close to the present work (13.97%). This shows the variety of bacteria isolated from different hospitals and different periods of time and depends on many factors.
During the study, it was noticed that most of investigated hospital wards were colonized by mentioned bacteria, though the highest colonization was belonged to general ICU and NICU. This is a matter of concern, since the patients hospitalized in these units are seriously ill or due to age or immunological status are more prone to get infections. Both isolated Gram negative bacteria are responsible for serious infections. In case of Enterobacter spp., it may cause infections including bacteremia, lower respiratory tract, skin and soft-tissue infections (17). Besides, P. aeruginosa as a main opportunistic pathogen comprises potential capacity to cause nosocomial infections which affects a remarkable number of patients in ICU. The importance of this bacterium is that it shows a high antibiotic resistance, so it is able to cause severe infections in critically ill patients associated with substantial morbidities and mortality (18, 19). Subsequently in the present study P. aeruginosa isolates were highly antibiotic resistant and apart from other antibiotics, showed 60.4% resistance to Carbapenem antibiotics. This was higher than the rate reported by Gladestone et al. for this bacterium in their study (20). Colonization of this organism in different parts of hospitals is a common concern worldwide, and there are reports of severe infections caused by highly antibiotic resistant P. aeruginosa strains in ICU and other wards of hospitals (18, 21).
In the latter study, the origin of the organism was the water outlets. In present study, enterobacter was the most prevalent isolate with relatively high antibiotic resistance. This finding was similar to other studies in which drug resistant Gram negative bacteria has been reported to isolate frompatients in ICUs (21). In a recent study, non fermenting bacteria such as acinetobacter spp. were among the isolates from ICU patients (20), while no acinetobacter was isolated from samples in this study. Based on investigations, the potential factors enhancing the emergence of resistant bacteria in hospitalized patients are mainly duration of stay in intensive care wards, using mechanical devices, prior antibiotics use, especially broad-spectrum drugs such as third-generation cephalosporin, fluoroquinolone, and/or imipenem (22). Many patients in the present work had a history of such antibiotics consumption.
In summary, the findings of this study indicate the emergence of antibiotic resistant infections in our hospital wards especially ICU and NICU and there is still a need to improve the effectiveness of integrated infection control programs focused on HAI surveillance in teaching teaching hospitals under investigation to control and manage nosocomial infections caused by highly resistant organisms.